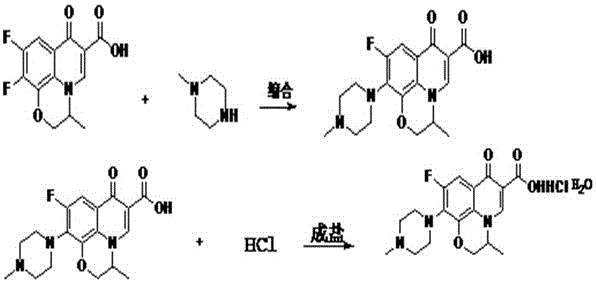
Preparation method of levofloxacin hydrochloride
A technology of levofloxacin hydrochloride and levofloxacin carboxylic acid, which is applied in the field of pharmaceutical preparation, can solve the problems of low purity of levofloxacin hydrochloride, long process flow, and difficult operation, etc., and achieve the effects of shortening the process cycle, avoiding high temperature conditions, and reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A preparation method of levofloxacin hydrochloride, comprising the following steps;
[0031] 1) Mix and dissolve 50 g of levofloxacin, 100 g of N-methylpiperazine and 30 g of dimethyl sulfoxide, then stir and raise the temperature in a water bath to 80°C for 7 hours, then recover dimethyl sulfoxide under reduced pressure to dryness , lower the dry matter to room temperature and add 400g of chloroform and 100g of water to stir, and adjust the pH value to 7.0 with ammonia water, and separate the layers after standing still for 1 hour, discard the water layer, take the chloroform layer and add 75g of water, wash thoroughly for 30 minutes, and then separate the layers , take the chloroform layer and back-extract the water layer with 50 g of chloroform and incorporate it into the obtained chloroform layer, then distill the chloroform layer under normal pressure to dryness, and then distill it under reduced pressure to a solid powder.
[0032] 2) Add 60g of purified water to ...
Embodiment 2
[0034] A preparation method of levofloxacin hydrochloride, comprising the following steps;
[0035] 1) Mix and dissolve 50g of levofloxacin, 85g of N-methylpiperazine and 30g of dimethyl sulfoxide, then stir and raise the temperature in a water bath to 85°C for 6 hours, then recover dimethyl sulfoxide under reduced pressure to dryness , lower the dry matter to room temperature and add 400g of chloroform and 100g of water to stir, and adjust the pH value to 7.0 with ammonia water, and separate the layers after standing for 1 hour, discard the water layer, take the chloroform layer and add 100g of water, wash thoroughly for 30 minutes, and then separate the layers , take the chloroform layer and back-extract the water layer with 50 g of chloroform and incorporate it into the obtained chloroform layer, then distill the chloroform layer under normal pressure to dryness, and then distill it under reduced pressure to a solid powder.
[0036] 2) Add 60g of purified water to the solid...
Embodiment 3
[0038] A preparation method of levofloxacin hydrochloride, comprising the following steps;
[0039] 1) Mix and dissolve 50g of levofloxacin, 85g of N-methylpiperazine and 30g of dimethyl sulfoxide, then stir and raise the temperature in a water bath to 90°C for 8 hours, then recover dimethyl sulfoxide under reduced pressure to dryness , lower the dry matter to room temperature and add 400g of chloroform and 110g of water to stir, and adjust the pH value to 7.0 with ammonia water, and separate the layers after standing still for 1 hour, discard the water layer, take the chloroform layer and add 80g of water, wash fully for 30 minutes, and then stand still for layering , take the chloroform layer and back-extract the water layer with 60 g of chloroform and incorporate it into the obtained chloroform layer, then distill the chloroform layer under normal pressure to dryness, and then distill it under reduced pressure to a solid powder.
[0040] 2) Add 60g of purified water to the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com