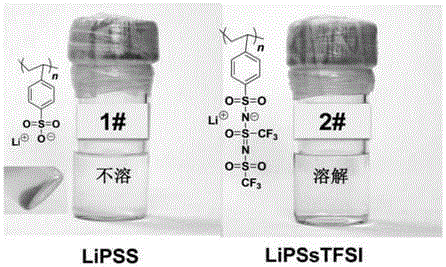
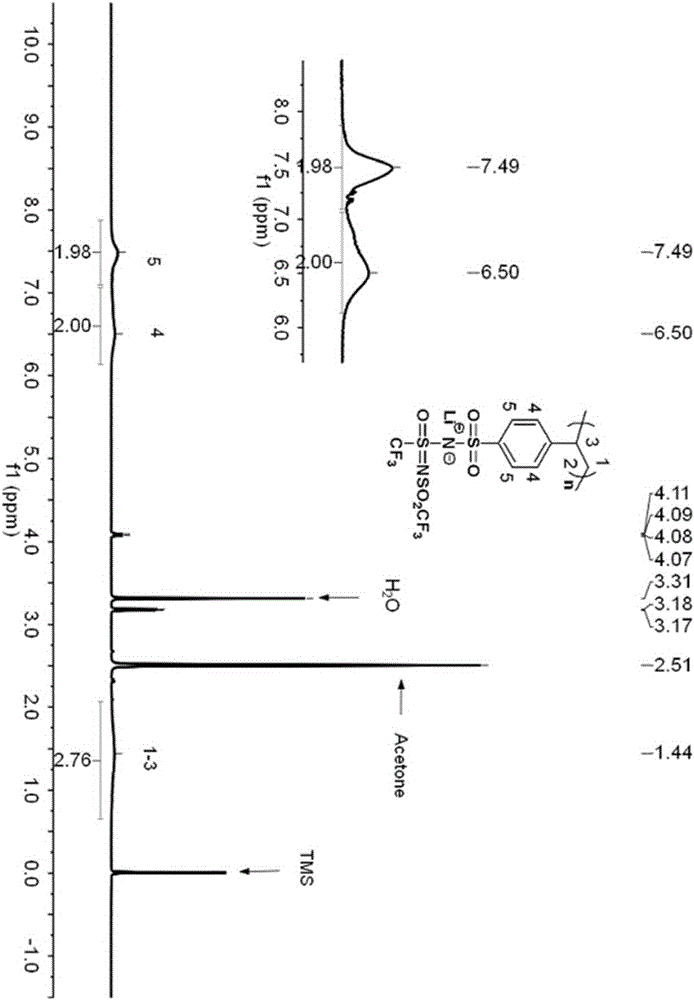
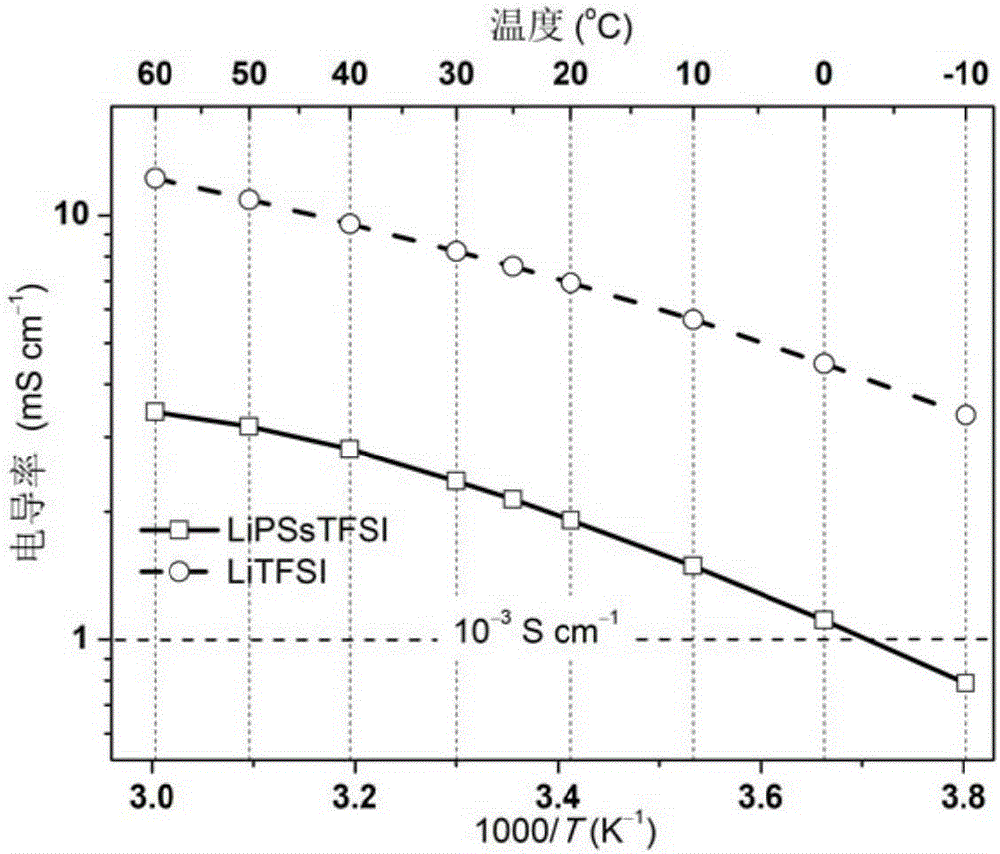
Iamine-polyanion lithium salt and preparation method thereof as well as application of iamine-polyanion lithium salt as nonaqueous electrolyte
A polyanion and electrolyte technology, which is used in the application field of polyanion lithium salt as an electrolyte material in lithium ion batteries and secondary lithium batteries, can solve the problem of poor compatibility of metal lithium, concentration polarization, poor solubility, and inability to meet the requirements of two Lithium-ion battery application requirements and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~6
[0056] Embodiments 1-6 relate to the preparation of polyanionic lithium salts containing "S-fluoroalkylsulfonylimide group"
Embodiment 1
[0057] Embodiment 1: the preparation of poly (p-styrenesulfonyl) (trifluoromethyl (S-trifluoromethylsulfonyl) sulfonyl) imide lithium salt
[0058] Add 3.00 g (6.63 mmol) (p-styrenesulfonyl) (trifluoromethyl(S-trifluoromethylsulfonimido)sulfonyl)imide lithium (LiSsTFSI) monomer to a 25 mL reaction flask, 0.021 g (0.13 mmol) of azobisisobutyronitrile (AIBN) and 5.0 g of refined acetonitrile. Pass argon to exhaust oxygen for 2h, and then react for 10h at 60°C under the protection of argon. After the reaction is completed, cool to room temperature, and slowly add the reaction solution dropwise to excess toluene under stirring to precipitate a colloidal solid, pour out the upper liquid, and repeat the dissolution and precipitation three times to obtain a gel-like Solid; vacuum-dry the viscous polymer at 80°C for 8 hours to obtain lithium poly(p-styrenesulfonyl)(trifluoromethyl(S-trifluoromethylsulfonylimide)sulfonyl)imide (LiPSsTFSI), yield 2.14 g (4.71 mmol), yield 71%.
Embodiment 2
[0059] Embodiment 2: Preparation of poly(p-styrenesulfonyl) (trifluoromethyl (S-trifluoromethylsulfonyl) sulfonyl) imide potassium salt
[0060] Add 4.11 g (8.48 mmol) (p-styrenesulfonyl) (trifluoromethyl (S-trifluoromethylsulfonimide) sulfonyl) potassium imide (KSsTFSI) monomer to a 25 mL reaction flask, 0.028 g (0.17 mmol) of azobisisobutyronitrile (AIBN) and 5.4 g of refined DMF. Pass argon to exhaust oxygen for 2h, and then react for 10h at 60°C under the protection of argon. After the reaction was completed, cool to room temperature, drop the reaction solution dropwise into rapidly stirred excess anhydrous diethyl ether (100mL), make it precipitate into a viscous solid, pour out the diethyl ether in the upper layer slowly, dissolve in this way, Precipitate three times to obtain a gel-like solid; the viscous polymer was vacuum-dried at 80°C for 8 hours to obtain poly(p-styrenesulfonyl)(trifluoromethyl(S-trifluoromethylsulfonylimide) )sulfonyl)imide potassium salt (KPSsTF...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com