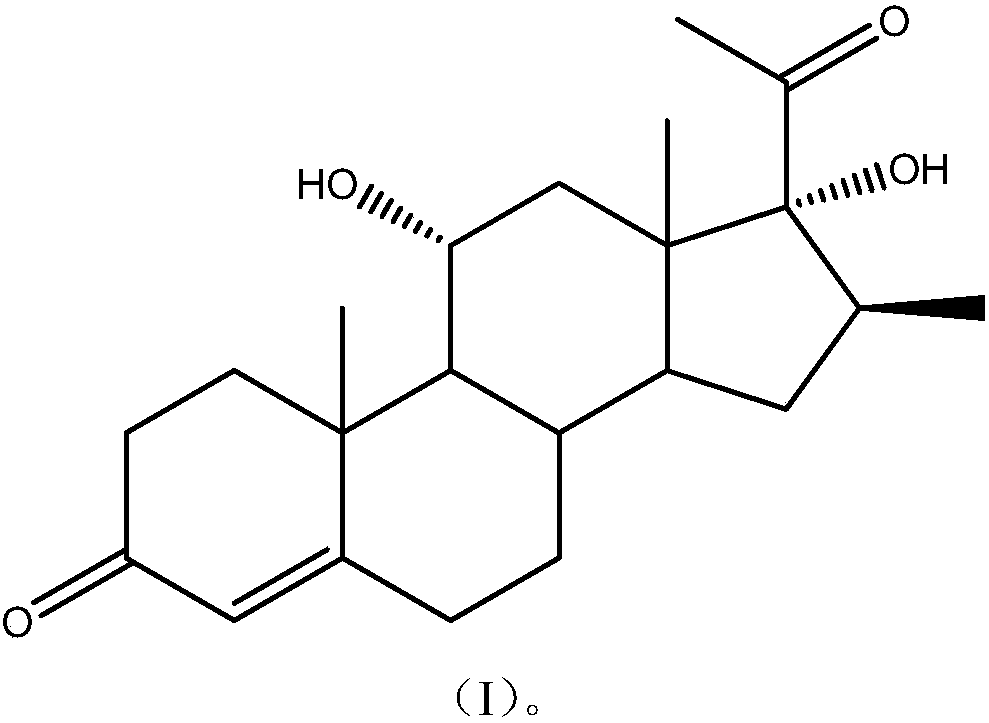
16-beta methylpregnene steroid hormone intermediate and preparation method of 16-beta methylpregnene steroid hormone intermediate
A technology of methylpregnene steroids and intermediates, which is applied in the field of 16-beta methylpregnene steroid hormone intermediates and their preparation, can solve the problems of long synthesis route and high cost of intermediates, and achieves reduction of three wastes "Effects of emissions, reduced consumption, shortened synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、16
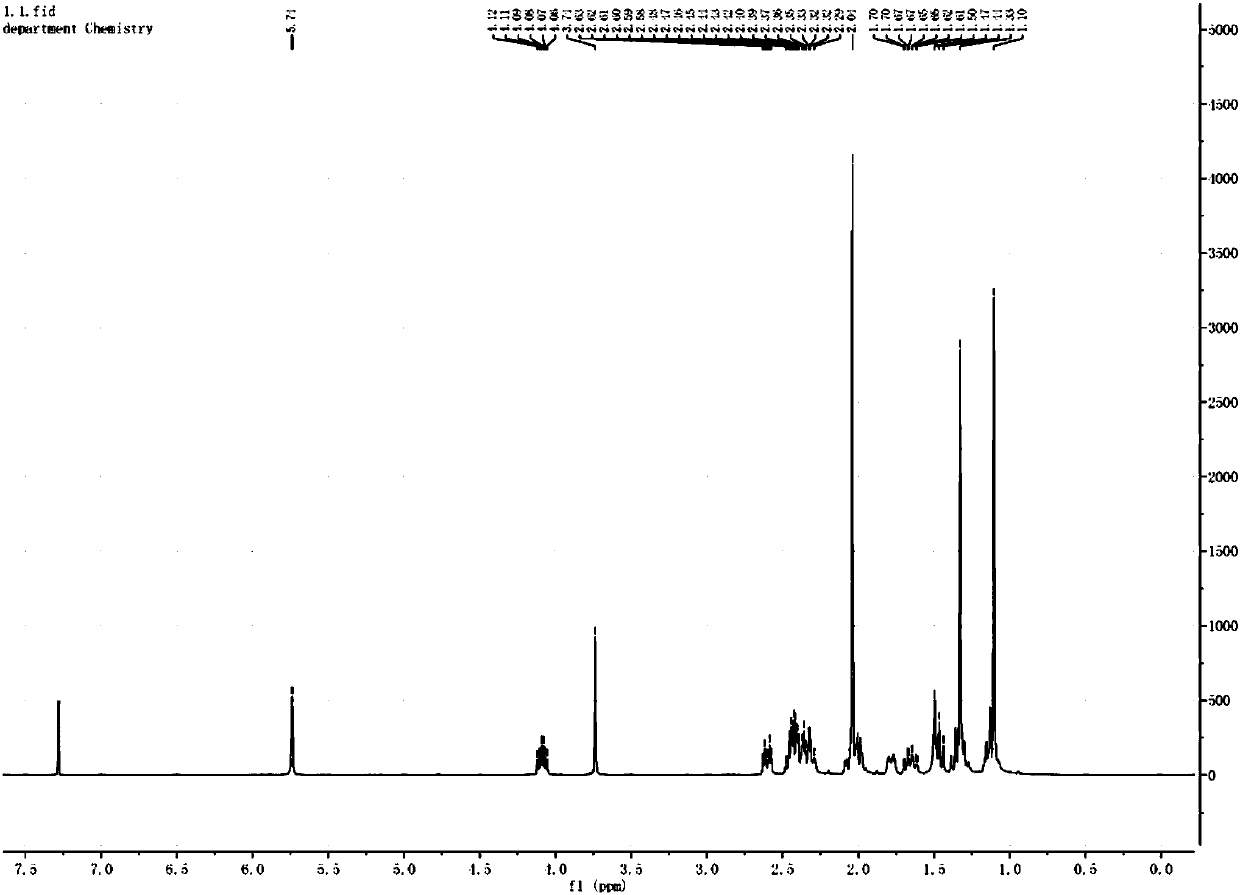
[0071] The preparation of embodiment 1,16-beta methylpregnene steroid hormone intermediate compound (I)
[0072] (1) Ketal reaction:
[0073] Add 10 g of compound (II) to 100 g of tetrahydrofuran, add 0.01 g of concentrated sulfuric acid and 15 g of ethylene glycol, and stir the reaction at 20°C for 4 hours; concentrate under reduced pressure to remove the organic solvent, add methanol for recrystallization, and obtain compound (III);
[0074]
[0075] (2) Grignard reaction:
[0076] Add 10 g of compound (III) to 20 g of the Grignard reaction solution, heat to reflux for 25 hours, cool down to room temperature, pour the reaction solution into a saturated ammonium chloride solution, stir and analyze in water for 2 hours to obtain compound (IV);
[0077]
[0078] (3) Hydrolysis reaction
[0079] Add 10 g of compound (IV) to 20 g of glacial acetic acid, heat to reflux for 30 minutes, cool to room temperature, and pour the reactant into water to obtain compound (I).
[00...
Embodiment 2、16
[0082] The preparation of embodiment 2, 16-beta methylpregnene steroid hormone intermediate compound (I)
[0083] (1) Ketal reaction:
[0084] Add 10 g of compound (II) to 200 g of chloroform, add 0.01 g of p-tolueneboronic acid and 20 g of ethylene glycol, and stir and react at 50°C for 4 hours; concentrate under reduced pressure to remove the organic solvent, add ethanol for recrystallization, and obtain Compound (III);
[0085] (2) Grignard reaction:
[0086] Add 10 g of compound (III) to 15 g of Grignard reaction solution, heat to reflux for 25 hours, cool down to room temperature, pour the reaction solution into a saturated ammonium chloride solution, stir and analyze in water for 2 hours to obtain compound (IV);
[0087] (3) Hydrolysis reaction
[0088] Add 10 g of compound (IV) to 50 g of glacial acetic acid, heat to reflux for 30 minutes, cool to room temperature, and pour the reactant into water to obtain compound (I).
[0089] The overall yield of compound (I) wa...
Embodiment 3、16
[0090] The preparation of embodiment 3, 16-beta methylpregnene steroid hormone intermediate compound (I)
[0091] (1) Ketal reaction:
[0092] Add 10g of compound (II) to 30g of dichloromethane, add 0.01g of phosphorus trichloride and 20g of ethylene glycol, stir and react at 30°C for 4 hours; concentrate under reduced pressure to remove the organic solvent, dichloromethane, add propanol for re- crystallization to obtain compound (III);
[0093] (2) Grignard reaction:
[0094] Add 10 g of compound (III) to 30 g of the Grignard reaction solution, heat to reflux for 25 hours, cool down to room temperature, pour the reaction solution into a saturated ammonium chloride solution, stir and analyze in water for 2 hours to obtain compound (IV);
[0095] (3) Hydrolysis reaction
[0096] Add 10 g of compound (IV) to 30 g of glacial acetic acid, heat to reflux for 30 minutes, cool to room temperature, and pour the reactant into water to obtain compound (I).
[0097] The overall yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com