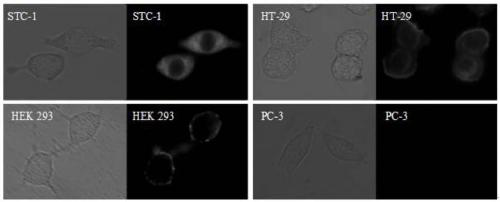
A kind of gpr120 small molecule fluorescent probe and its application
A fluorescent probe, small molecule technology, used in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
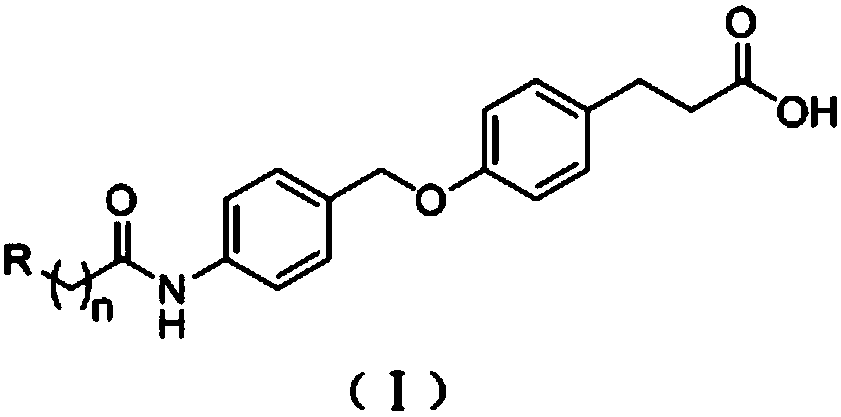
[0059] Embodiment 1: Preparation of naphthalene diimide fluorophore probes (L1-L3):
[0060]
[0061] Concrete synthetic route is as follows:
[0062]
[0063] Preparation of Intermediate 1
[0064] In a 100mL round-bottomed flask, add p-hydroxyphenylpropionic acid (1.5g, 9.03mmol) and anhydrous methanol (35mL) and stir to dissolve, then add a catalytic amount of concentrated sulfuric acid (15 drops), and stir at reflux at 60°C for 6 Hours later, it was lowered to room temperature, the reaction liquid was spun out, added water and dichloromethane for extraction, the organic layers were combined, washed with saturated brine, anhydrous MgSO 4 Dry overnight, filter, concentrate, and purify by column chromatography to obtain 1.54 g of a colorless transparent oil with a yield of 95%. 1 H NMR (400MHz, DMSO): δ9.15(s, 1H), 6.99(d, J=8.4Hz, 2H), 6.65(d, J=8.5Hz, 2H), 3.57(s, 3H), 2.73( t, J=7.6Hz, 2H), 2.54(t, J=7.6Hz, 2H); EI-MS: ([M] + ): 180.1.
[0065] Preparation of in...
Embodiment 2
[0090] Embodiment 2: Preparation of coumarin-based fluorophore probes (L4-L7):
[0091]
[0092] Concrete synthetic route is as follows:
[0093]
[0094] Preparation of Intermediate 7
[0095] In a 100mL round bottom flask, add 4-(diethylamino) salicylaldehyde (1.5g, 7.76mmol) and 40mL of absolute ethanol, then add diethyl malonate (1.24g, 7.76mmol), stir to make Dissolve morpholine (68 mg, 0.78 mmol) and acetic acid (20 μL) in 2 mL of absolute ethanol to obtain a mixed solution, then add the mixed solution to the above reaction solution, reflux and stir for 24 h, cool the reaction solution at ice temperature, and The reaction solution was spin-dried to obtain intermediate 7, which was directly used in the next step without purification.
[0096] Preparation of intermediate 8
[0097] Dissolve intermediate 7 (400mg, 1.38mmol) in a small amount of methanol solution, add 25mL of 2M NaOH solution, stir at room temperature for 24 hours, adjust the pH with 1M HCl to make th...
experiment example 1
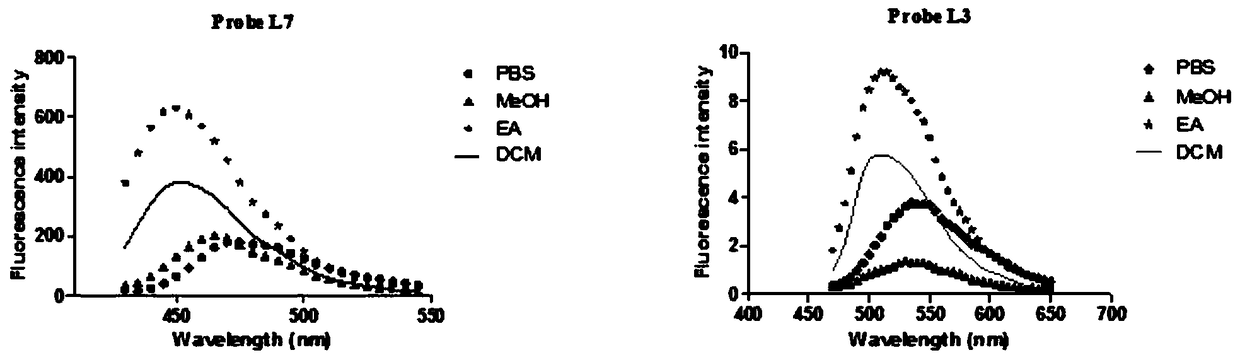
[0139] Experimental Example 1: Determination of Optical Activity
[0140] Table 1: Optical properties of probe molecules
[0141]
[0142]
[0143] Note: All the above optical properties were measured in Tris-HCl buffer at pH=7.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com