Polysaccharide derivative containing polypeptide targeting factor and preparation method thereof
A polysaccharide derivative and peptide targeting technology, applied in the field of biomedical materials, can solve the problems of destroying the activity of polypeptides and polysaccharides, destroying the activity of polypeptides and polysaccharides, and failing to achieve the expected goals, so as to achieve cheap equipment and raw materials, reduce toxic and side effects, The effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 1. Preparation method
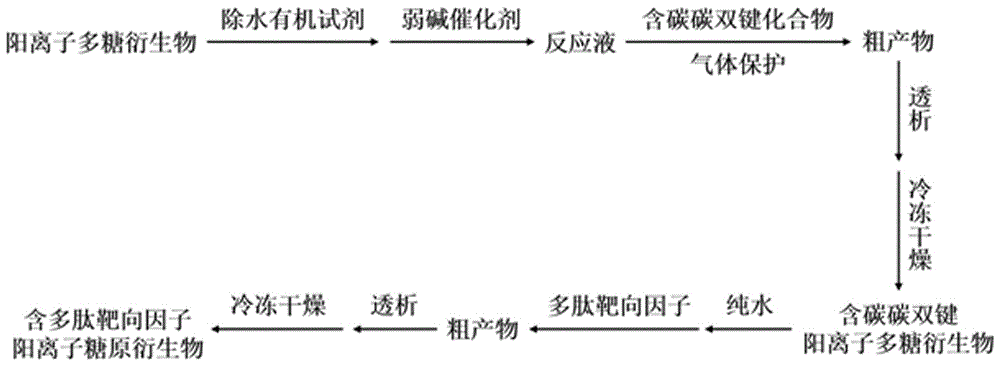
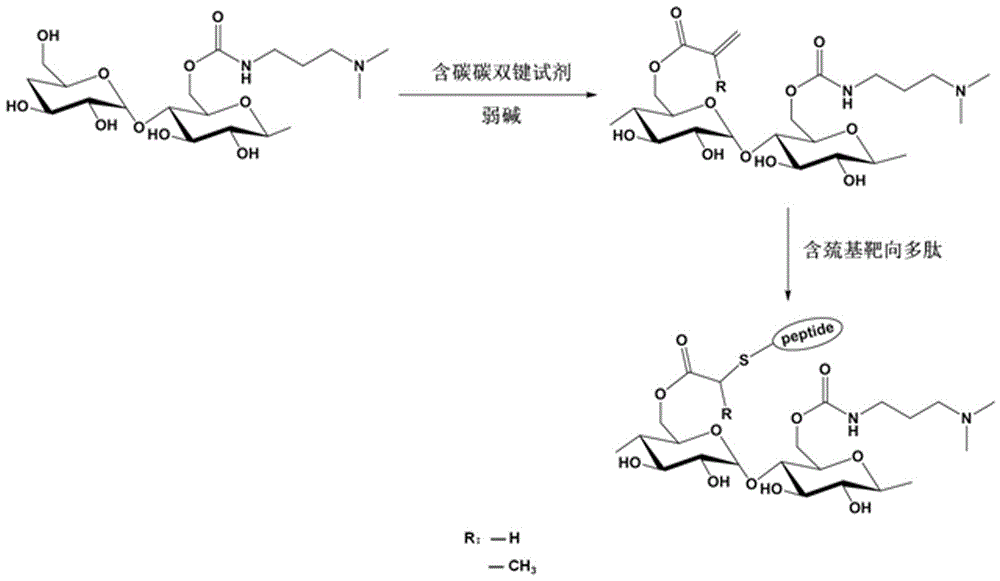
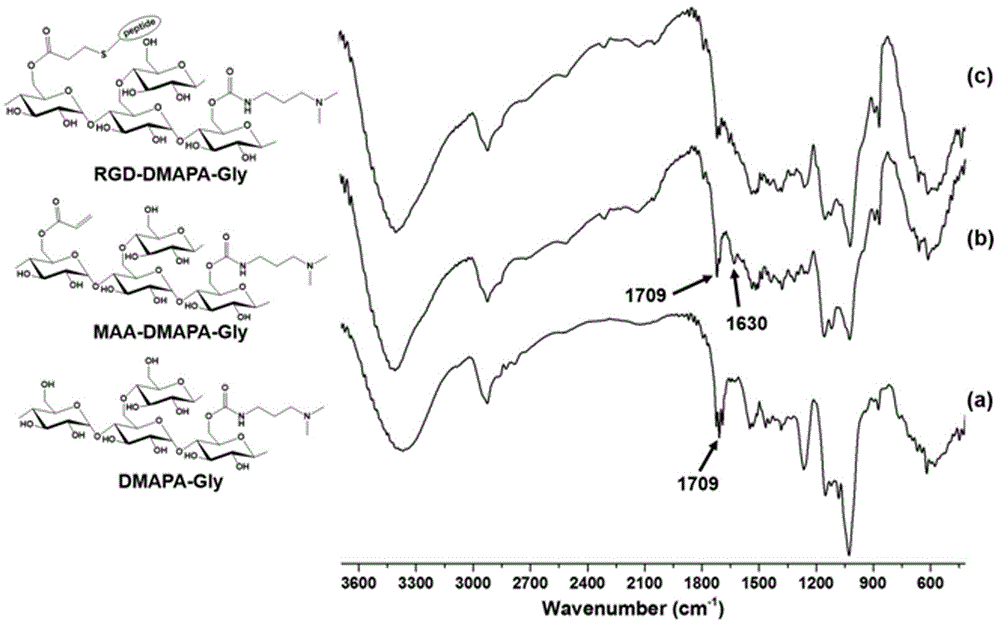
[0053] The process flow diagram and synthetic route diagram for preparing polysaccharide derivatives containing polypeptide targeting factors in the present invention are respectively as follows figure 1 and figure 2 shown.
[0054] The specific preparation method is as follows:
[0055] (1) Weigh 0.1g of 3-dimethylaminopropylaminoglycogen derivative (DMAPA-Gly) into a dry flask, add 10mL of dehydrated dimethylformamide, and stir at room temperature at 200r / min under nitrogen protection 12 hours to dissolve.
[0056] (2) Slowly add 0.5 mL of pyridine dropwise to the solution obtained in step (1) with a syringe, stir at room temperature at 200 r / min for 10 minutes, then slowly add 0.5 mL of acrylic acid with a syringe, and stir at room temperature at 200 r / min under the protection of nitrogen to react 24 Hour.
[0057] (3) The reaction solution was dialyzed against deionized water for 1 day, and then freeze-dried to obtain a 3-dimethylaminop...
Embodiment 2
[0070] 1. Preparation method
[0071] (1) Weigh 0.4g of 3-dimethylaminopropylamino chitosan derivative into a dry flask, add 5mL of dehydrated dichloromethane, and stir at room temperature at 300r / min under nitrogen protection for 24 hours to dissolve.
[0072] (2) Slowly add 0.1 mL of triethylamine dropwise to the solution obtained in step (1) with a syringe, stir at room temperature at 300 r / min for 20 minutes, then slowly add 0.1 mL of acrylic anhydride with a syringe, under nitrogen protection, stir at room temperature at 300 r / min The reaction was stirred for 1 hour.
[0073] (3) The reaction solution was dialyzed against deionized water for 2 days, and then freeze-dried to obtain 3-dimethylaminopropylamino chitosan derivatives containing acrylic acid groups.
[0074] (4) Weigh 0.1 g of 3-dimethylaminopropylamino chitosan derivative containing acrylic acid group into a dry flask, add 10 mL of pure water, stir at 300 r / min at room temperature for 1 hour to dissolve.
[0...
Embodiment 3
[0080] 1. Preparation method
[0081] (1) Weigh 1 g of 3-dimethylaminopropylamino pullulan derivative into a dry flask, add 10 mL of dehydrated dimethylformamide, and stir at room temperature at 500 r / min for 2 hours under the protection of argon to dissolve.
[0082](2) Slowly add 1.0 mL of pyridine dropwise to the solution obtained in step (1) with a syringe, stir at room temperature at 500 r / min for 30 minutes, then slowly add 1.0 mL of methacrylic acid with a syringe, under the protection of argon, stir at room temperature at 500 r / min The reaction was stirred for 12 hours.
[0083] (3) The reaction solution was dialyzed against deionized water for 3 days, and then freeze-dried to obtain 3-dimethylaminopropylamino pullulan derivatives containing methacrylic acid groups.
[0084] (4) Weigh 0.2 g of 3-dimethylaminopropylamino pullulan derivative containing methacrylic acid group into a dry flask, add 15 mL of pure water, stir at 500 r / min at room temperature for 12 hours to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com