Preparation method for octreotide
A technology of octreotide and chromatography, applied in the field of biopharmaceuticals, can solve the problems of dilute sample concentration, low yield, and large volume, and achieve the effect of optimizing the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 (HPLC method detects octreotide precursor crude product and product solution purity after purification)
[0027] Instrument: Waters 2695 / 2489 high performance liquid chromatography
[0028] Separation column: Kromasil 100-3.5C18column 4.6×100mm
[0029] Mobile phase: A is 10% tetramethylammonium hydroxide: water: acetonitrile volume percentage is 2:88:10 aqueous solution, B is 10% tetramethylammonium hydroxide: water: acetonitrile volume percentage is 2:38:60 of aqueous solution.
[0030] Flow rate is 0.8mL / min, detection wavelength is 210nm, room temperature detection,
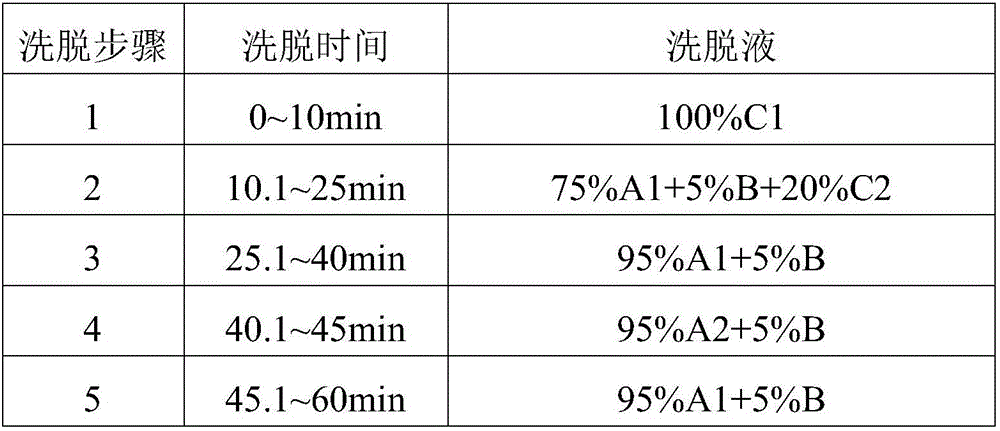
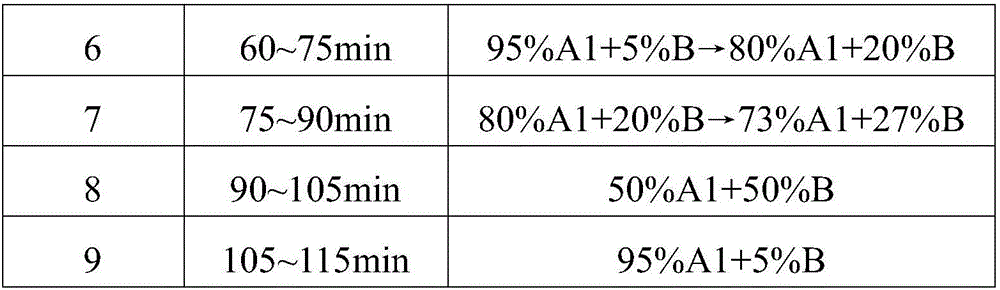
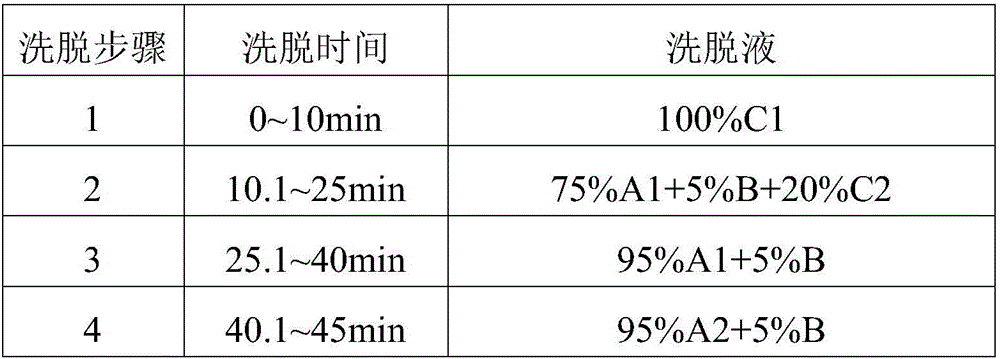
[0031] The elution gradient is shown in the table below, and the percentages are volume percentages.
[0032] mobile phase elution gradient
[0033] Elution step Elution time eluent 1 0~30min 73%A+27%B 2 30~31min 73%A+27%B→55%A+45%B 3 31~37min 73%A+27%B
Embodiment 2
[0034] Embodiment 2 (75mm inner diameter L&L4003 preparative column packing)
[0035] Using Load&Lock dynamic axial compression and static locking technology, the filler is styrene-divinylbenzene copolymer (reversed phase packing Agilent PLRP-S), the pore size is 10nm, the particle size is 10μm, and the column packing density is 0.33g / mL. Bed pressure 650psi, using Varian chromatography packing system, 370g dry powder filler, 2L methanol after stirring and homogenizing, poured into the L&L4003 preparative column with an inner diameter of 75mm, the compression ratio is 3:1, and the carrier gas is N 2 , adjust the carrier gas pressure so that the pressure of the oil pressure gauge is 2000psi, and the dynamic axial compression to the height of the column bed is 26cm, which is used as the preparative column for the reverse-phase cyclization, reverse-phase purification and reverse-phase desalination schemes.
Embodiment 3
[0036] Example 3 Reverse-phase cyclization, reverse-phase purification and reverse-phase desalting of octreotide precursor crude product raw material
[0037] Instrument: Varian SD-1 high pressure liquid phase preparation system
[0038] Chromatographic column: self-packed preparative column Load&Lock4003 75×260mm in Example 2, PLRP-S 10μm 10nm
[0039] The crude product of octreotide precursor is the crude product of octreotide precursor obtained by cleavage and drying by solid phase synthesis, and its structural formula is D-phenylalanyl-L-cysteinyl-L-phenylalanyl-D-tryptophanyl- Trifluoroacetate salt of L-lysyl-L-threonyl-L-cysteinyl-L-threoninol.
[0040] The crude octreotide precursor solution is a 5 g / L solution formed by dissolving the crude octreotide precursor in 5% acetonitrile aqueous solution.
[0041] Mobile phase A1 is pure water, A2 is 0.02% H by volume 2 o 2 pH is the NaOH aqueous solution of 7.5, and mobile phase B is acetonitrile, and mobile phase C1 is d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com