Preparation method of swine H1N1 subtype influenza virus hemagglutinin recombinant protein and liquid phase chip detection kit for virus antibody
A swine influenza virus and recombinant protein technology, which is applied in the preparation of swine H1N1 subtype influenza virus hemagglutinin recombinant protein, and in the field of liquid chip detection kits for the virus antibody, can solve the problems of cumbersome operation, long cycle time, and poor sensitivity , to achieve the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Construction of embodiment 1 expression plasmid
[0043] 1. Extraction of influenza virus total RNA and synthesis of cDNA
[0044] Refer to the instructions of the RNA extraction kit to extract the total viral RNA. After the total RNA was obtained, the reverse transcription operation was performed referring to the instruction manual of the M-MLV reverse transcriptase of Treasure Bioengineering (Dalian) Co., Ltd. The reaction system is shown in Table 1.
[0045] Reagent name volume RNA 9.5μL 5×MLV Buffer 4.0 μL MLV reverse transcriptase 1.0 μL dNTPs (2.5mmol each) 4.0 μL RNase Inhibitor (20U / μL) 0.5μL reverse transcription primer 1.0 μL total 20 μL
[0046] The reagents in Table 1 were added and mixed thoroughly, and placed in a water bath at 42°C for 1 hour, and the obtained cDNA product was stored at -20°C for future use.
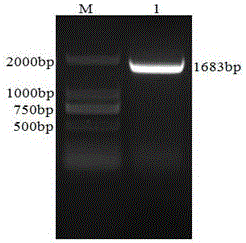
[0047] 2. Amplification of SIV H1N1 HA gene
[0048] Using Oligo7.0 biological ...
Embodiment 2
[0055] Expression, purification and identification of embodiment 2 recombinant protein
[0056] Rosetta (DE3) Escherichia coli containing the recombinant plasmid pMAL-cHA was inoculated into Luria-Bertani (LB) liquid culture fluid, and when the culture OD value reached 0.6, adding a final concentration of 0.3 mM isopropylthiogalactoside ( IPTG), after incubating at 37°C for 2 hours, the product was collected for SDS-PAGE detection; Western blot method was used to detect the expressed product with anti-SIV HA monoclonal antibody as the primary antibody. At the same time, the bacterial cells were collected by centrifugation, ultrasonically disrupted in an ice bath, and after centrifugation again, the precipitate and supernatant were tested by SDS-PAGE to analyze the solubility of the expression product.
[0057] The size of the HA target protein was in line with expectations, and there was a band at about 100KD ( image 3 ), and the target protein was expressed in both the supe...
Embodiment 3
[0063] Example 3 Application of Rosetta-pMAL-cHA to establish a liquid phase chip detection method
[0064] 1. Coupling of antigens to microspheres
[0065] Refer to Luminex's Technical encyclopedia, two-step amide reaction: first, the phosphorylated microspheres are activated by disodium hydrogen phosphate buffer, Sulfo-NHS and EDC solution, and then the HA recombinant protein prepared in Example 2 is used as an antigen to form a covalent Amide bonds, microspheres coupled with antigens were resuspended in PBS-TBN solution and stored at 4°C in the dark. The specific operation steps are as follows:
[0066] (1) Vortex the microsphere suspension for 1 min to disperse the microspheres evenly;
[0067] (2) Transfer 100 μL of microspheres to a 1.5mL centrifuge tube, centrifuge at 8000g / min for 2min, place on a magnetic stand, and gently remove the supernatant (do not suck away the microspheres);
[0068] (3) Add 100μL ddH 2 O, vortex for 1min, then centrifuge at 8000g / min for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dilution degree | aaaaa | aaaaa |
| Dilution degree | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com