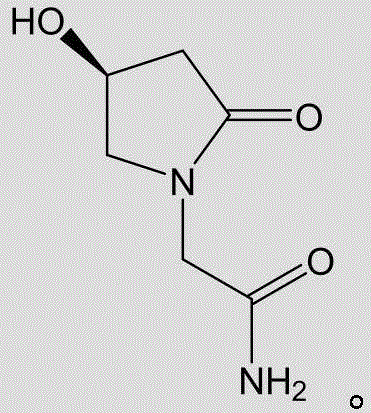
L-oxiracetam oral membrane and preparation method thereof
One kind of oral film and another technology are applied in the field of preparation of levoxiracetam oral film, which can solve the problems of low drug loading, solid particle coalescence and enlarged liposome preparation process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
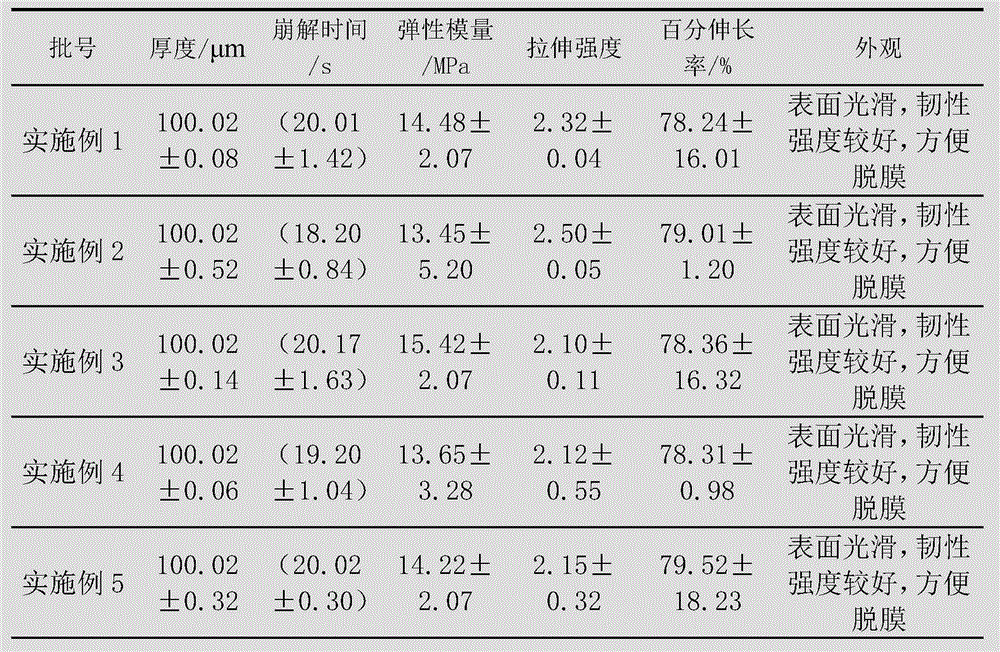
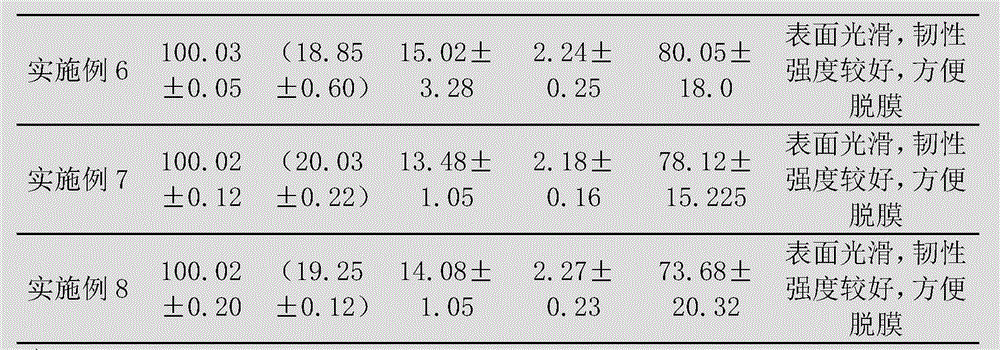
Embodiment 1
[0087] The preparation of levoxiracetam oral film adopts the following steps:
[0088] 1) Dissolve 65g of film-forming material (composed of maltodextrin and hydroxypropyl cellulose, wherein the dosage of hydroxypropyl cellulose is 30g) with 80mL of deionized water, and remove air bubbles to obtain a uniform viscous liquid;
[0089] 2) Disperse 25g of glycerin, 15g of microcrystalline cellulose, 4g of citric acid and 2g of sucralose with 50mL of deionized water to form a dispersion;
[0090] 3) Add the dispersion of step 2) to the viscous liquid of step 1), and add 18g of levoxiracetam to disperse evenly, and then let it stand to remove air bubbles;
[0091] 4) Coat the viscous liquid after removing air bubbles with a drug film coating dryer at a coating speed of 60cm / min, then dry at 75-77°C, and peel off.
Embodiment 2
[0093] The preparation of levoxiracetam oral film adopts the following steps:
[0094] 1) Dissolve 40g of film-forming material (composed of maltodextrin and hydroxypropyl cellulose, wherein the amount of hydroxypropyl cellulose is 18g) with 50mL of deionized water, and remove air bubbles to obtain a uniform viscous liquid;
[0095] 2) Disperse 15g of propylene glycol, 10g of low-substituted hydroxypropyl cellulose, 2g of malic acid and 2g of acesulfame K with 60mL of deionized water to form a dispersion;
[0096] 3) Add the dispersion of step 2) to the viscous liquid of step 1), and add 18g of levoxiracetam to disperse evenly, then let it stand to remove air bubbles;
[0097] 4) Coat the viscous liquid after removing air bubbles with a drug film coating dryer at a coating speed of 85cm / min, then dry at 70-72°C, and peel off.
Embodiment 3
[0099] The preparation of levoxiracetam oral film adopts the following steps:
[0100] 1) Dissolve 50g of film-forming material (composed of maltodextrin and hydroxypropyl cellulose, wherein the amount of hydroxypropyl cellulose is 25g) with 50mL of deionized water, and remove air bubbles to obtain a uniform viscous liquid;
[0101] 2) Disperse 15g of triethyl citrate, 19g of microcrystalline cellulose, 3g of malic acid and 1g of aspartame with 40mL of deionized water to form a dispersion;
[0102] 3) Add the dispersion of step 2) to the viscous liquid of step 1), and add 15g of levoxiracetam to disperse evenly, then let it stand to remove air bubbles;
[0103] 4) Coat the viscous liquid after removing air bubbles with a drug film coating dryer at a coating speed of 70cm / min, then dry at 83-85°C, and peel off.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com