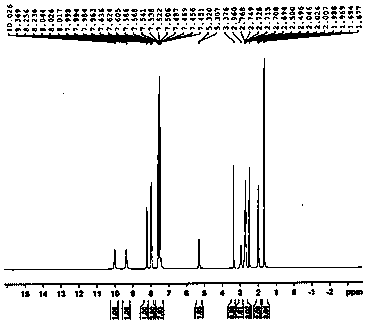
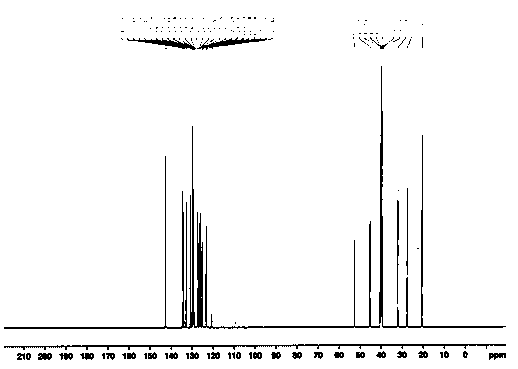
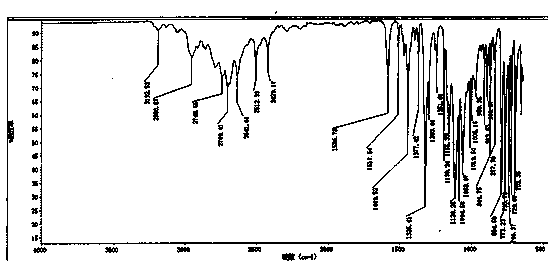
A kind of preparation method of cinacalcet hydrochloride
A technology of cinacalcet hydrochloride and ethyl acetate, which is applied in the field of drug synthesis, can solve the problems of high production cost and high price, and achieve the effects of less impurities, high economic value and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation method of cinacalcet hydrochloride in the present embodiment comprises the following steps
[0038] (1) Add 450 ml ethyl acetate, 0.20 mol (about 45.0 g) m-bromotrifluoromethylbenzene (II), 0.20 mol (about 4.8 g) magnesium powder, 0.002 mol (about 0.5 g) iodine to a 1000 ml reaction bottle granules, slowly heated to 55°C, kept stirring for 8 hours, stopped heating, slowly cooled to 0-10°C, slowly added 0.20mol (about 18.2g) of acryloyl chloride (Ⅲ) dropwise, and the dropwise addition was completed in 60 minutes.
[0039] After the dropwise addition, stir overnight at 0~10°C, slowly add 180ml of ice-water mixture, stir for 30 minutes, transfer the reaction solution into a 1000ml separatory funnel, discard the incompletely dissolved solids, let the mixture stand for 15 minutes, and separate the liquids. The lower aqueous phase was discarded.
[0040] Add 180ml of purified water into the separatory funnel, oscillate, shake well, let stand for 15 minutes, s...
Embodiment 2
[0052] The preparation method of cinacalcet hydrochloride in the present embodiment comprises the following steps
[0053] (1) Add 450 ml tetrahydrofuran to a 1000ml reaction bottle, add 0.20mol (about 45.0g) m-bromotrifluoromethylbenzene (II), 0.213mol (about 5.1g) magnesium powder, 0.00236mol (about 0.6g) iodine particles , slowly heated to 50~60°C, kept warm and stirred for 8h to generate Grignard's reagent, then stopped heating, slowly cooled to 0~10°C, slowly added 0.212mol (about 19.2g) of acryloyl chloride (Ⅲ) dropwise, and the dropwise addition was completed in 60 minutes .
[0054] After the dropwise addition, stir overnight at 0~10°C. The next day, slowly add 180ml of ice-water mixture, stir for 30 minutes, transfer the reaction solution into a 1000ml separatory funnel, discard the incompletely dissolved solids, let the mixture stand for 15 minutes, separate the liquids, and discard the lower aqueous phase.
[0055] Add 180ml of purified water into the separatory f...
Embodiment 3
[0062] The preparation method of cinacalcet hydrochloride in the present embodiment comprises the following steps
[0063] (1) Add 450 ml of diethyl ether, 0.20 mol (about 45.0 g) of m-bromotrifluoromethylbenzene (II), 0.242 mol (about 5.8 g) of magnesium powder, and 0.00256 mol (about 0.65 g) of iodine particles into a 1000 ml reaction bottle, Slowly heat to 55°C, keep stirring for 8 hours, stop heating, slowly cool down to 0-10°C, slowly add 0.23mol (about 20.9g, 18.8ml) of acryloyl chloride (Ⅲ) dropwise, and dropwise complete in 60 minutes.
[0064] After the dropwise addition, stir overnight at 0~10°C. The next day, slowly add 180ml of ice-water mixture, stir for 30 minutes, transfer the reaction solution into a 1000ml separatory funnel, discard the incompletely dissolved solids, let the mixture stand for 15 minutes, separate the liquids, and discard the lower aqueous phase.
[0065] Add 180ml of purified water into the separatory funnel, oscillate, shake well, let stand ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com