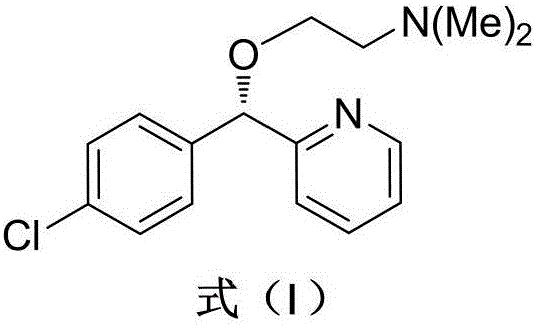
Asymmetric synthesis method for anti-allergy drug carbinoxamine
A synthesis method and technology of carbisamin, applied in the direction of organic chemistry, etc., can solve the problems of limited industrial application, difficult to obtain, expensive catalyst, etc., and achieve the effects of mild conditions, convenient operation and low price.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
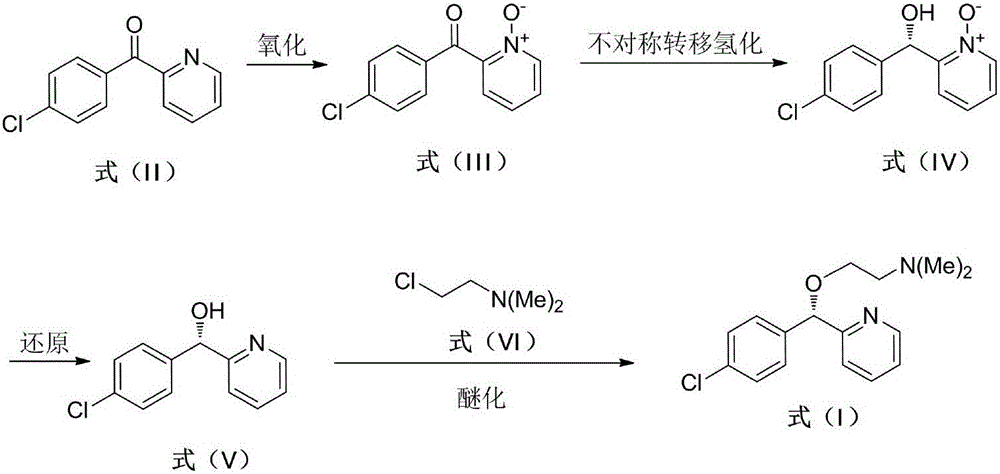
[0028] Embodiment 1: Synthesis of (4-chlorophenyl) (2-pyridyl) ketone-N-oxide (formula (III))
[0029] Dissolve 2.0g of (4-chlorophenyl)(2-pyridyl)methanone in 15mL of hydrogen peroxide (30% aqueous solution), add 5mL of acetic acid, heat to 85°C for 12h, and check the progress of the reaction by TLC until the raw materials react Complete, add saturated sodium bicarbonate solution, extract with dichloromethane, wash, combine the organic phases, dry over anhydrous sodium sulfate, filter, and remove the solvent under reduced pressure to obtain 2.1 g of white solid with a yield of 95%. 1 H NMR (400MHz, CDCl 3 ):δ=7.46-7.50(m,5H),7.82(dt,J 1 =4.4Hz,J 2 =2.4Hz, 2H), 8.27-8.29 (m, 1H)ppm.
Embodiment 2-5
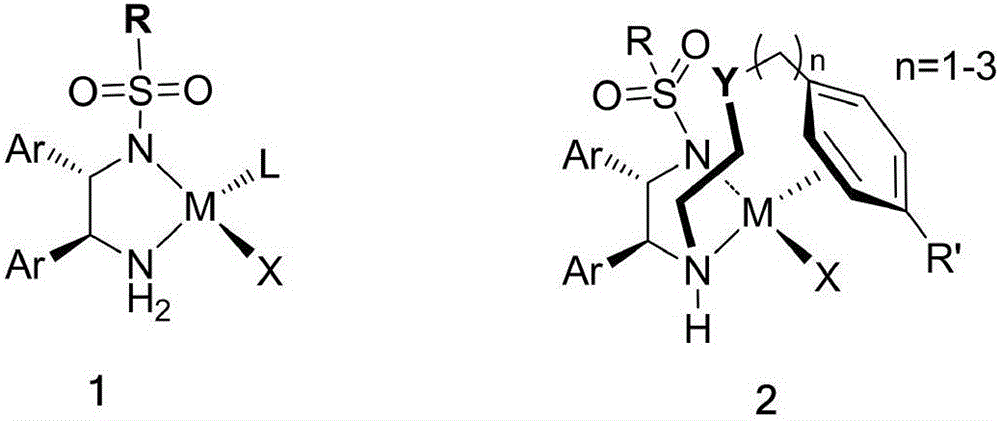
[0030] The catalyst structure used in embodiment 2-5:
[0031]
Embodiment 2
[0032] Embodiment 2: Synthesis of (S)-(4-chlorophenyl)(2-pyridyl)methanol-N-oxide (formula (IV))
[0033]
[0034] In a 20 mL Schlenk test tube, add 38 mg of catalyst 1a, 0.23 g of (4-chlorophenyl)(2-pyridyl)methanone-N-oxide, 1.6 g of sodium formate, seal the test tube, replace the gas with nitrogen 3 times, and add by syringe 5mL DMSO / H 2 O (1:1) mixed solvent, reacted for 24 hours at 50 DEG C, added water after the completion of the reaction, extracted 3 times with ethyl acetate, combined and concentrated to dryness, purified to obtain a white solid 0.208g, yield 90%, HPLC assay product ( The enantiomeric excess ee of S)-(4-chlorophenyl)(2-pyridyl)methanol-N-oxide was 95%. HPLC separation conditions: OD-H chiral column, mobile phase: n-hexane / isopropanol=90:10 (volume ratio), flow rate: 1.0mL / min, wavelength: 220nm, column temperature: 30°C, retention time: t R =9.0min,t S = 11.5min; 1 H NMR (400MHz, CDCl 3 ): δ=6.08(d, J=4.4Hz, 1H), 6.42(d, J=4.8Hz, 1H), 7.00(t, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com