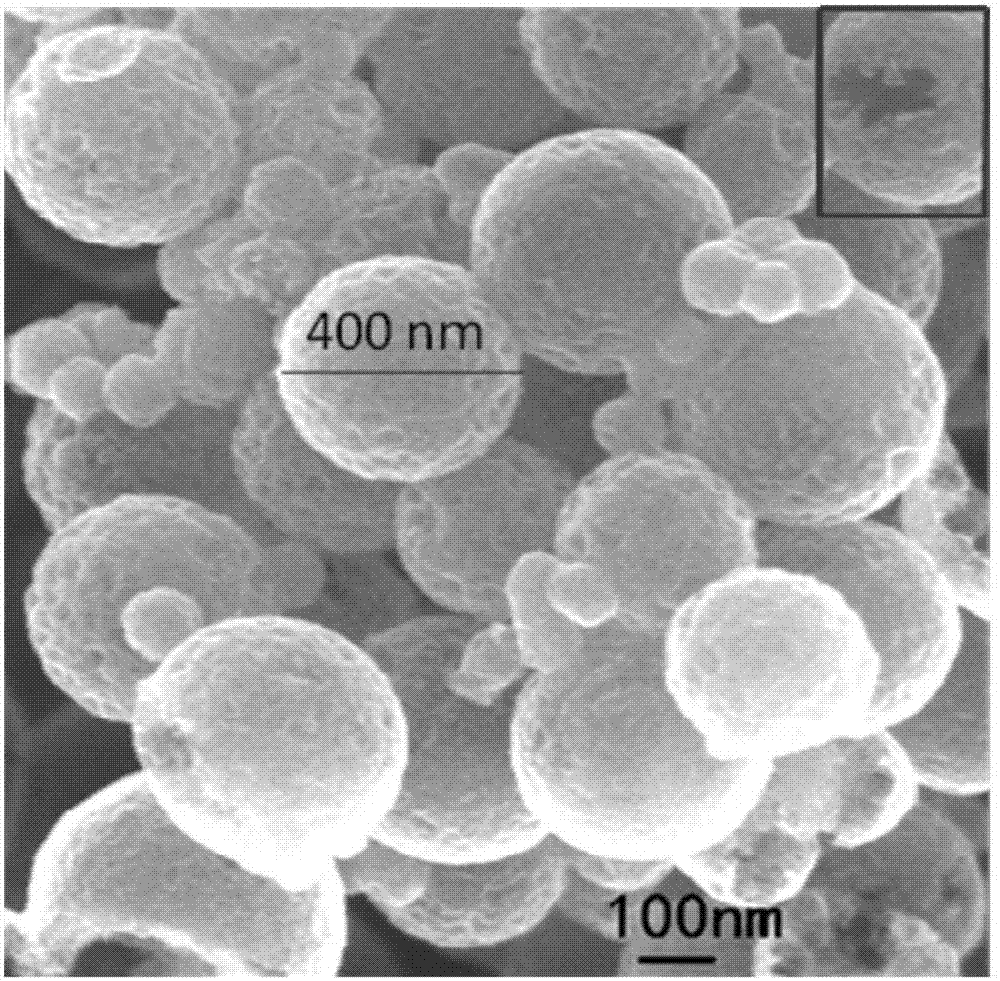
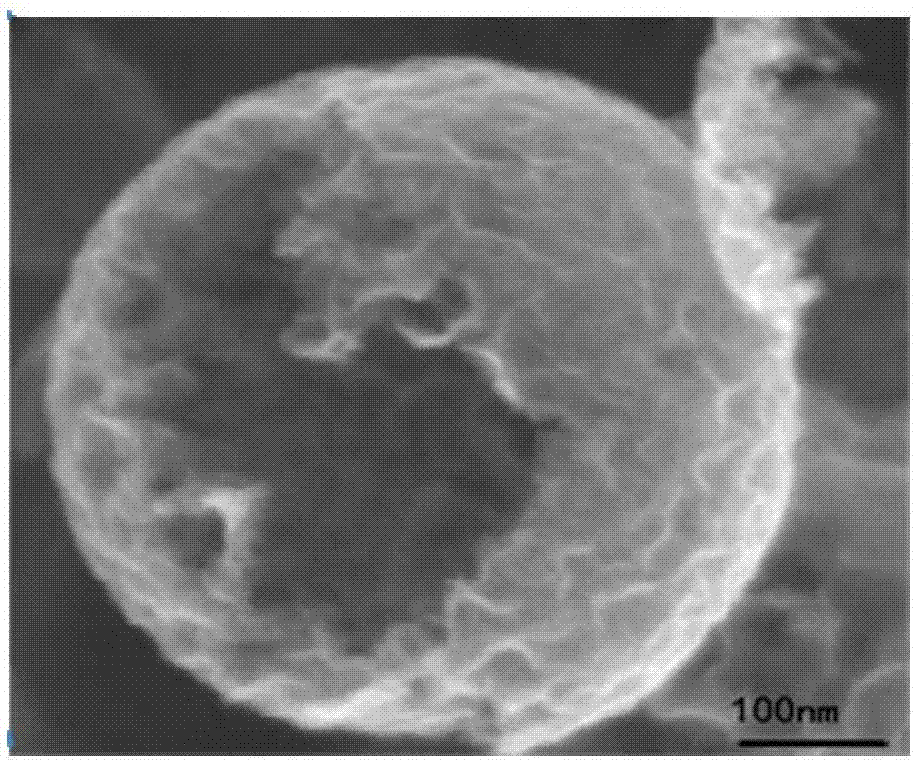
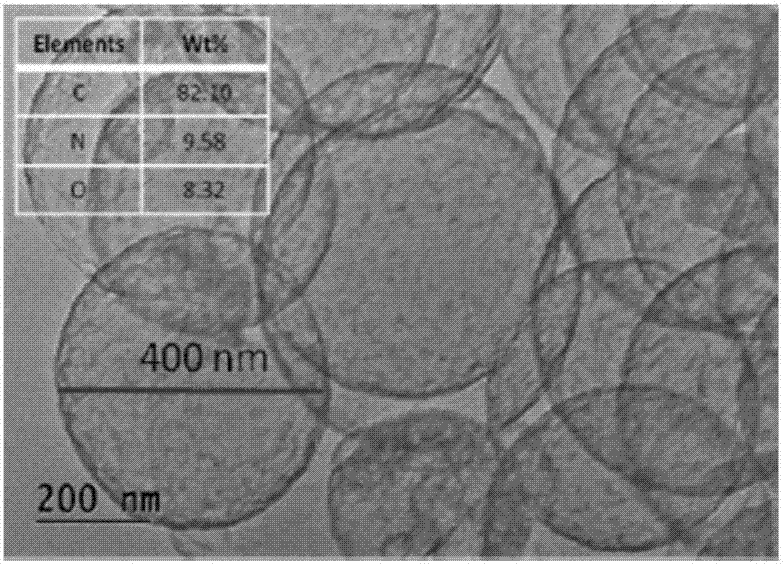
Nitrogen-doped porous hollow carbon sphere carbon dioxide adsorption material as well as preparation method and application thereof
A hollow carbon sphere, adsorption material technology, applied in chemical instruments and methods, alkali metal oxides/hydroxides, inorganic chemistry, etc., can solve the problem of difficult introduction of nitrogen and low introduction amount, low material recycling rate, adsorption capacity low problems, to achieve the effect of large adsorption capacity, controllable morphology, and high adsorption performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of SiO by the above stober method and solvothermal method 2 Ball flower:
[0038] Preparation of aqueous solution A: Add cetylpyridinium bromide (CPB) and urea (mass ratio 5:3) to an appropriate amount of deionized water, and vigorously stir at 500-700rmp at room temperature to make cetylpyridinium bromide (CPB) and urea are more evenly dispersed. Among them, the ratio of urea to water is 0.6g:30mL.
[0039] Prepare oil solution B: measure tetraethyl orthosilicate (TEOS), cyclohexane and n-pentanol (the volume ratio of tetraethyl orthosilicate, cyclohexane and n-pentanol is 9:100:5) to obtain oil solution B , add the oil solution B dropwise to the aqueous solution A under stirring at room temperature, and stir well at room temperature to obtain a microemulsion, and transfer the obtained microemulsion into a stainless steel high-pressure reactor with a polytetrafluoroethylene liner, at 120 ° C Solvent thermal crystallization for 20 hours, cooled to room te...
Embodiment 2
[0053] Preparation of SiO by the above stober method and solvothermal method 2 Ball flower:
[0054] Preparation of aqueous solution A: Add cetylpyridinium bromide (CPB) and urea (mass ratio: 5:3) to an appropriate amount of deionized water, and vigorously stir at 500rmp at room temperature to make cetylpyridinium bromide (CPB) ) and urea are dispersed more uniformly to obtain solution A. Among them, the ratio of urea to water is 0.6g:30mL.
[0055] Prepare oil solution B: measure tetraethyl orthosilicate (TEOS), cyclohexane and n-pentanol (the volume ratio of tetraethyl orthosilicate, cyclohexane and n-pentanol is 9:100:5) to obtain oil solution B , add the oil solution B dropwise to the aqueous solution A under stirring at room temperature, and stir well at room temperature to obtain a microemulsion, and transfer the obtained microemulsion into a stainless steel high-pressure reactor with a polytetrafluoroethylene liner, at 120 ° C Solvent thermal crystallization for 20 h...
Embodiment 3
[0059] Preparation of SiO by the above stober method and solvothermal method 2 Ball flower:
[0060] Preparation of aqueous solution A: Add cetylpyridinium bromide (CPB) and urea (mass ratio: 5:3) to an appropriate amount of deionized water, and vigorously stir at 700rmp at room temperature to make cetylpyridinium bromide (CPB) ) and urea are dispersed more uniformly to obtain solution A. Among them, the ratio of urea to water is 0.6g:30mL.
[0061] Prepare oil solution B: measure tetraethyl orthosilicate (TEOS), cyclohexane and n-pentanol (the volume ratio of tetraethyl orthosilicate, cyclohexane and n-pentanol is 9:100:5) to obtain oil solution B , add the oil solution B dropwise to the aqueous solution A under stirring at room temperature, and stir well at room temperature to obtain a microemulsion, and transfer the obtained microemulsion into a stainless steel high-pressure reactor with a polytetrafluoroethylene liner, at 120 ° C Solvent thermal crystallization for 20 h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com