Metallic nickel-nitrogen doped porous carbon materials, preparation method and application of metallic nickel-nitrogen doped porous carbon materials
A nitrogen-doped porous carbon and porous carbon material technology, applied in the field of nanomaterials, can solve the problems of thermodynamic instability, large surface energy and specific surface area, and reduced catalytic activity of metal nanomaterials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of Nickel Diacetyl Oxime:
[0037] (1) Prepare an aqueous solution of 0.1M nickel sulfate: take 13.14g of nickel sulfate hexahydrate solid and dissolve it completely in 500mL of water, stir evenly to prepare a 0.1M aqueous solution of nickel sulfate;
[0038] (2) Prepare 0.2M ethanol solution of dimethylglyoxime: take 11.6g of dimethylglyoxime and completely dissolve it in 500mL ethanol solution, stir evenly to prepare 0.2M ethanol solution of dimethylglyoxime;
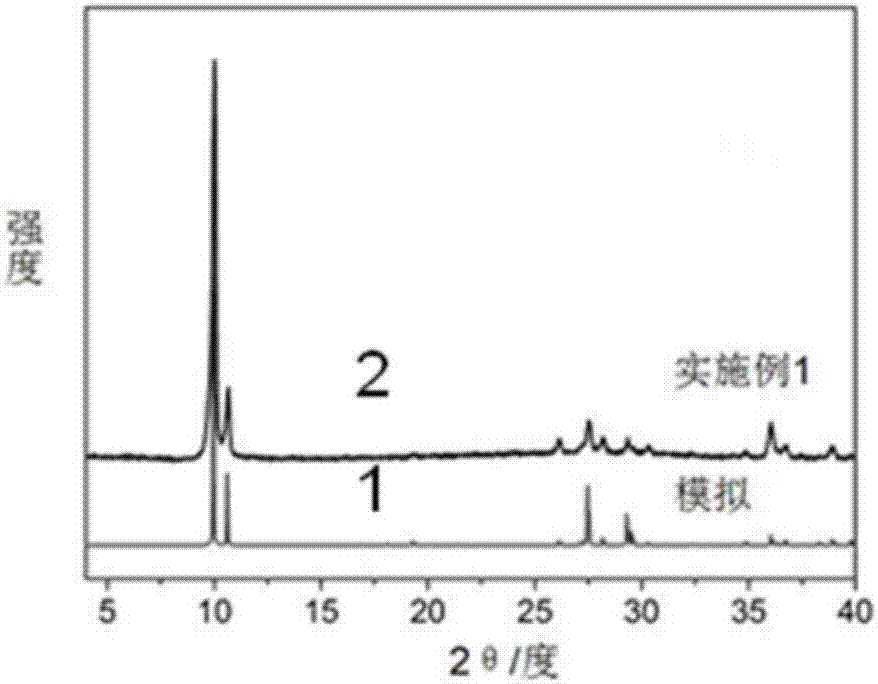
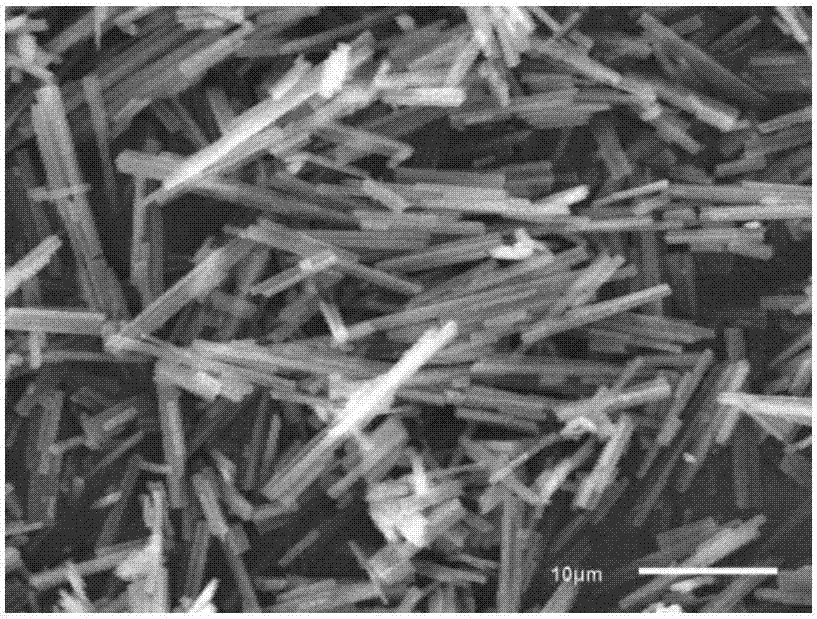
[0039] (3) Measure 28.7mL, 0.2M ethanol solution of dimethylglyoxime in a 50mL beaker, then dropwise add 10mL, 0.1M aqueous solution of nickel sulfate into the above-mentioned dimethylglyoxime solution, stir while adding dropwise, After all the addition was completed, the stirring was continued for 2 h, and a large amount of red precipitates appeared in the solution. The reaction solution was filtered, and the precipitate was washed 3 times with distilled water, then washed 3 times with ethanol sol...
Embodiment 2
[0042] Example 2: Using the dimethylglyoxime nickel obtained in Example 1 as a template to prepare metal nickel-nitrogen-doped porous carbon materials with different metal nickel contents
[0043] Prepare 3M HCl solution
[0044] Accurately measure 250mL of 12M HCl into a 1L volumetric flask, add distilled water to dilute to 1L to prepare a 3M HCl solution.
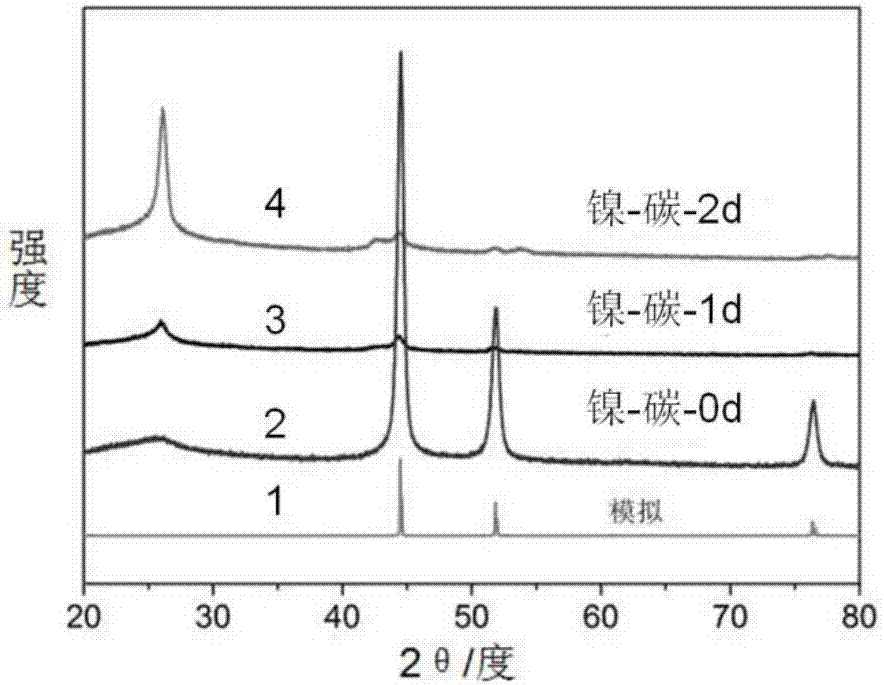
[0045]Take 100mg of nickel dimethylglyoxime and put it in a quartz crucible, spread it evenly, place the crucible in the middle of the carbonization furnace, first pass nitrogen gas for 1 hour, exhaust the air in the furnace to make it full of nitrogen gas, and then heat it at 5°C min -1 The heating rate was increased to 700°C. When the temperature reached the above temperature, the temperature was kept for 2 hours. After the temperature naturally dropped to room temperature, the crucible was taken out. The red sample on the crucible turned into a black sample, which was marked as nickel-carbon-0d.
[0046] Measure 2 par...
Embodiment 3
[0050] Example 3: Pore structure and microstructure analysis of metal nickel-nitrogen doped porous carbon material
[0051] Weigh 50 mg of the nickel-carbon-0d sample prepared in Example 2, heat to 200° C. under vacuum, activate for 5 hours, and then use an adsorption instrument to test its isothermal adsorption-desorption curve under liquid nitrogen conditions. Its adsorption-desorption isotherm curve is as follows: Figure 7 , is the adsorption curve of a typical mesoporous material, and the specific surface area of the sample is 256m 2 g -1 .
[0052] Take 0.001mg nickel-carbon-0d sample in a 1.5mL test tube, drop into ethanol, ultrasonically disperse on an ultrasonic instrument for 3min, and conduct transmission electron microscope (TEM) analysis. As shown in Figure 8, Figure 8a is a TEM image at low magnification, and it can be observed that elemental nickel particles (black particles) are wrapped in porous carbon; Figure 8b is a TEM image at high magnification, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com