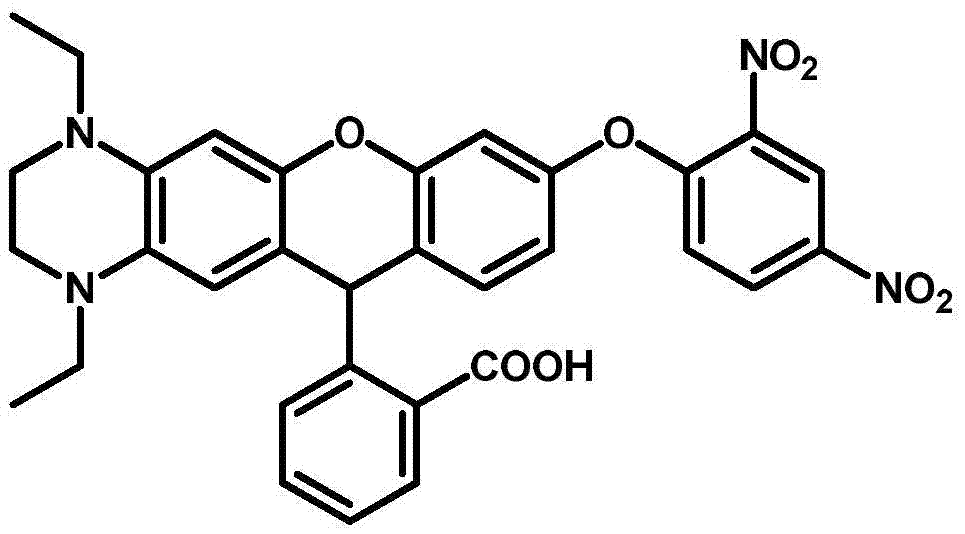
Fluorescent probe for detecting glutathione in blood, and synthesis method and application thereof
A technology of glutathione and fluorescent probes, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of non-specific recognition of GSH, achieve specific recognition ability, fast response speed, selective sex high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
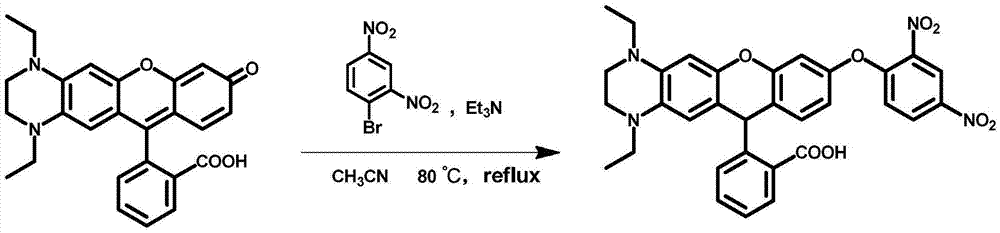
[0028] The following examples will further illustrate the present invention, but do not limit the present invention thereby. Example 1: Preparation of a fluorescent probe for detecting GSH in blood, the basic synthesis method is as follows:
[0029] In a 50mL single-necked bottle, add rhodamine fluorescein precursor compound (0.2mmol) and 2,4-dinitrobromobenzene 63mg (0.25mmol), add acetonitrile 10mL, triethylamine 0.5mL, heat and reflux at 80°C for 6h, after cooling Add 4 times the volume of dichloromethane, then extract 3 times with saturated saline, dry, filter, spin dry the solvent, pass through a silica gel column at room temperature, and elute under the condition of dichloromethane: anhydrous methanol = 20:1 The product was obtained in 70% yield.
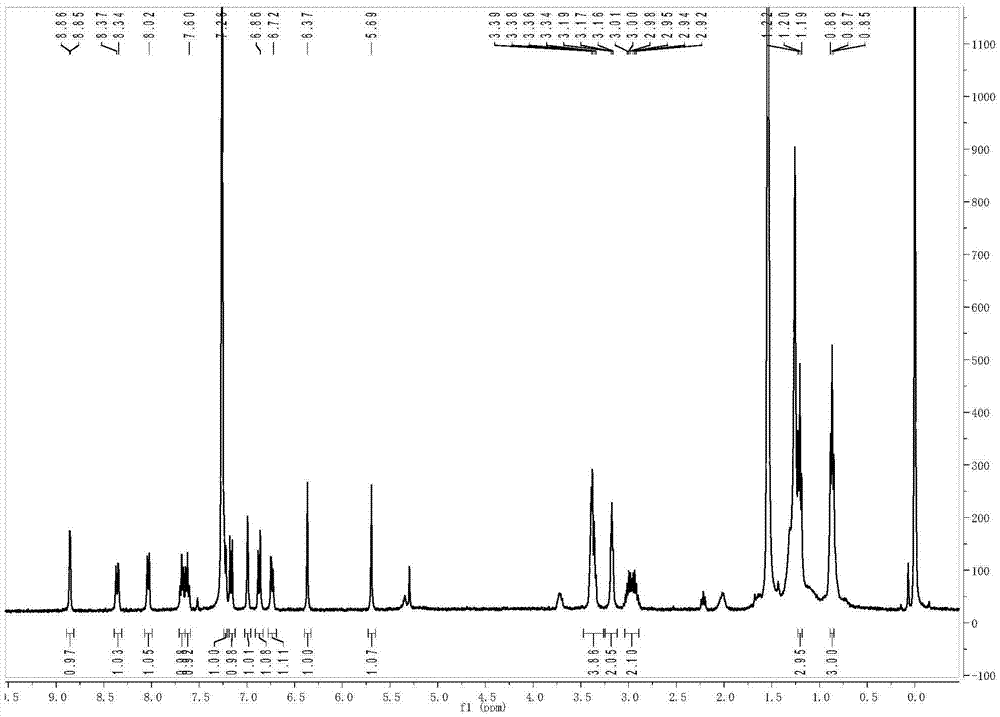
[0030] The hydrogen spectrum and carbon spectrum data of the probe molecule are as follows: 1 H NMR (400MHz, CDCl3) δ8.85 (d, J = 4Hz, 1H), 8.36 (dd, J = 9.2, 2.4Hz, 1H), 8.03 (d, J = 7.6Hz, 1H), 7.68 (t, J=7.2Hz, 1H), 7.62...
Embodiment 2
[0031] Embodiment 2: the response situation of the fluorescent probe prepared in embodiment 1 to GSH in the presence of GST
[0032] The probe was dissolved in DMSO solution to prepare 10 -3 Stock solution of M, in 4 mL, 20 mM pH=7.4
[0033] 1 μM probe, mixed solution of probe (1 μM) and GSH (5 μM), mixed solution of probe (1 μM) and GST (1 μM), probe (1 μM), GSH (5 μM) and GST were respectively configured in the PBS solution (1μM) mixed solution, measure the fluorescence to get Figure 5 .
[0034] Figure 5 In the presence of only the probe (1 μM), it showed weak fluorescence, and when only GSH (5 μM) was added to the probe solution, the fluorescence was only slightly enhanced. However, when GSH is added in the presence of 1 μM GST, the fluorescence is significantly enhanced, the enhancement is about 35 times, and the emission wavelength is in the near-infrared region (~700nm).
Embodiment 3
[0035] Embodiment 3: the selectivity of the fluorescent probe prepared in embodiment 1
[0036] Add 1 μM probe and 1 μM GST to 4 mL of 20 mM PBS solution with pH=7.4, then add 5 μM of 20 kinds of amino acids and GSH respectively, and measure the fluorescence to obtain Figure 6 , the results showed that the fluorescence was significantly enhanced only after the addition of GSH, indicating that this probe can specifically recognize GSH.
[0037] Add 1 μM probe, 1 μM GST to 4 mL, 20 mM PBS solution with pH=7.4, then add 5 μM GSH, Cys and Hcy respectively, and measure the fluorescence intensity to obtain Figure 7 . The results showed that the fluorescence was significantly enhanced only when GSH was added, which proved that the probe did not respond to the biosulfhydryl compounds Cys and Hcy that existed in large quantities in the organism, and further proved the selective recognition ability of the probe to GSH.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com