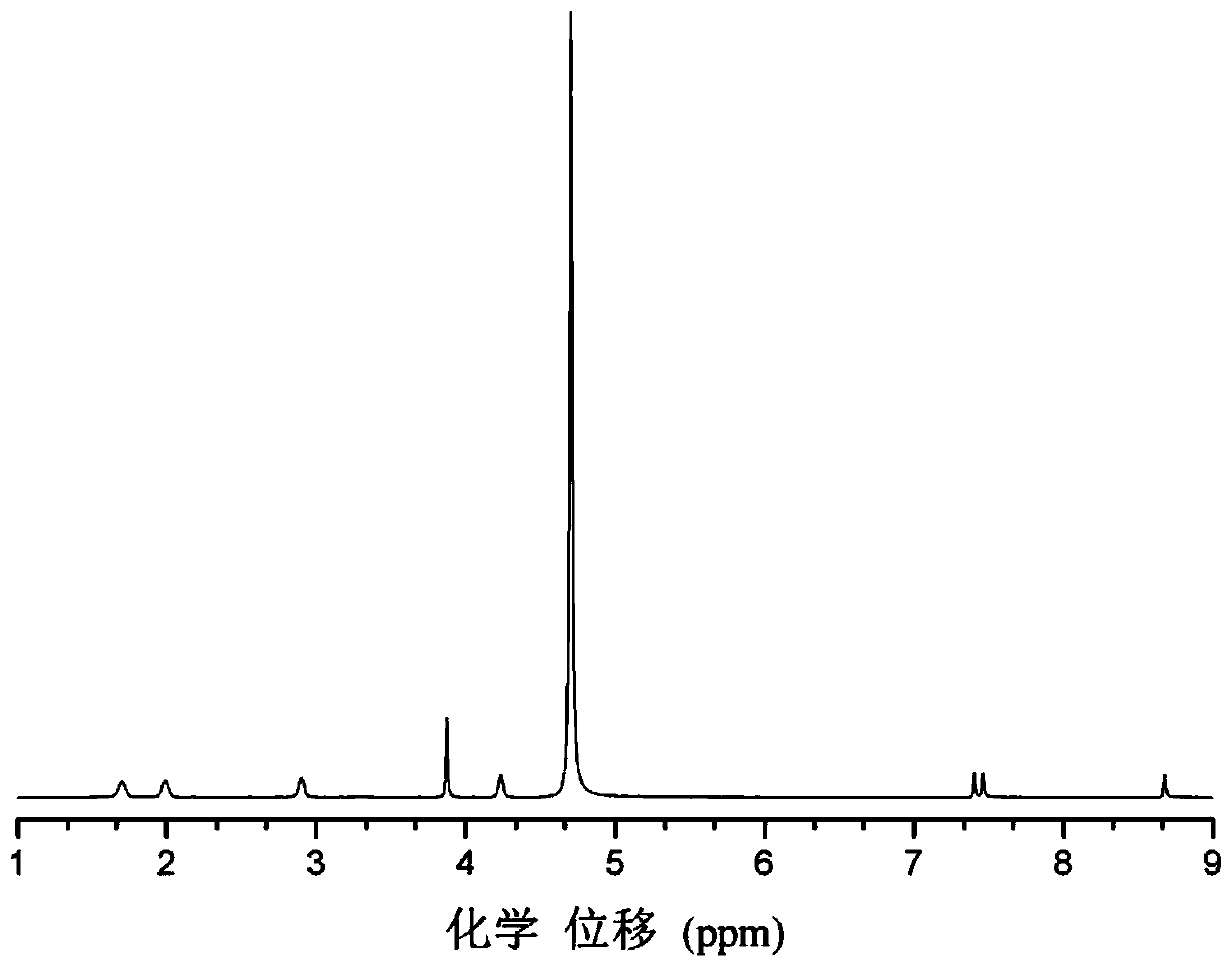
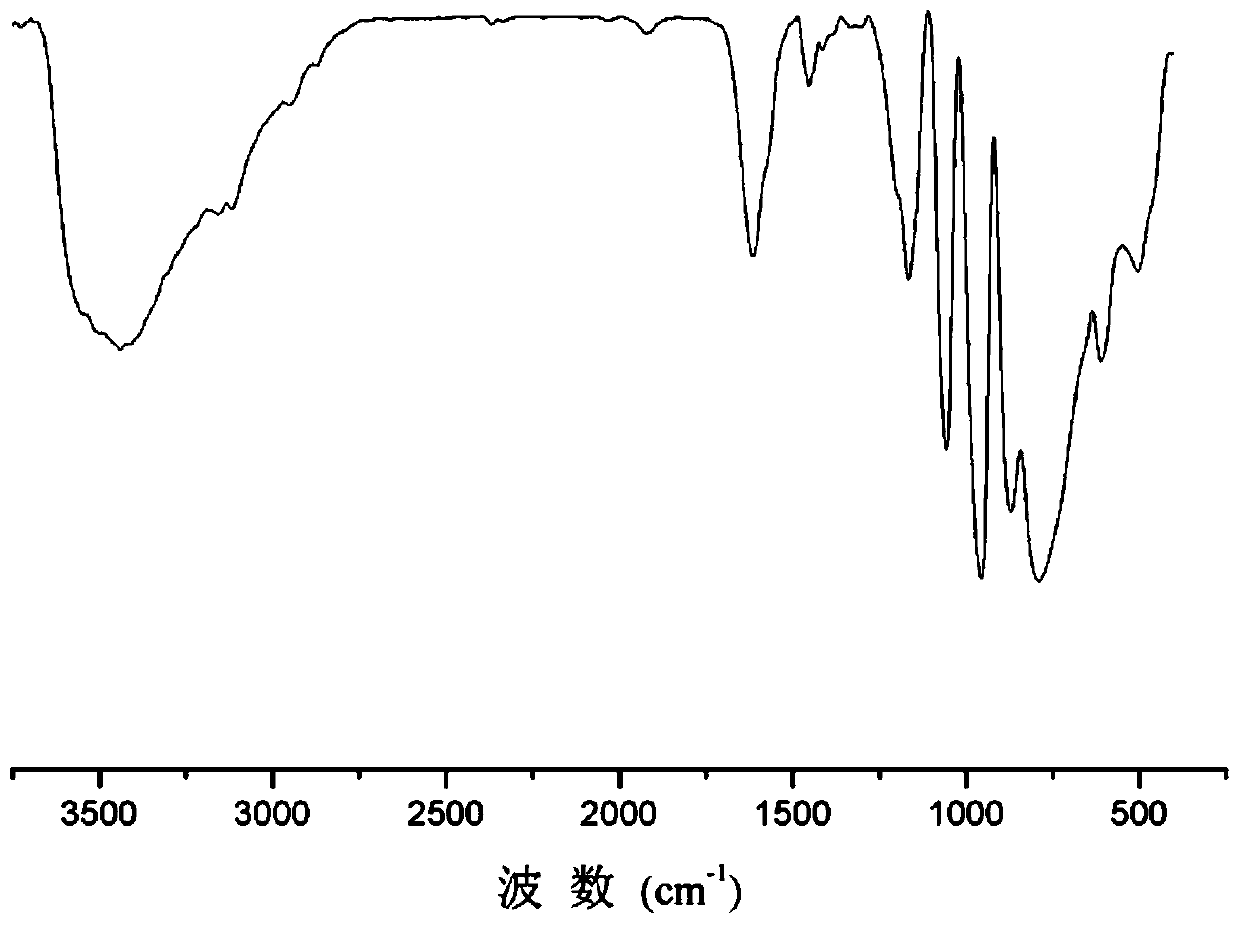
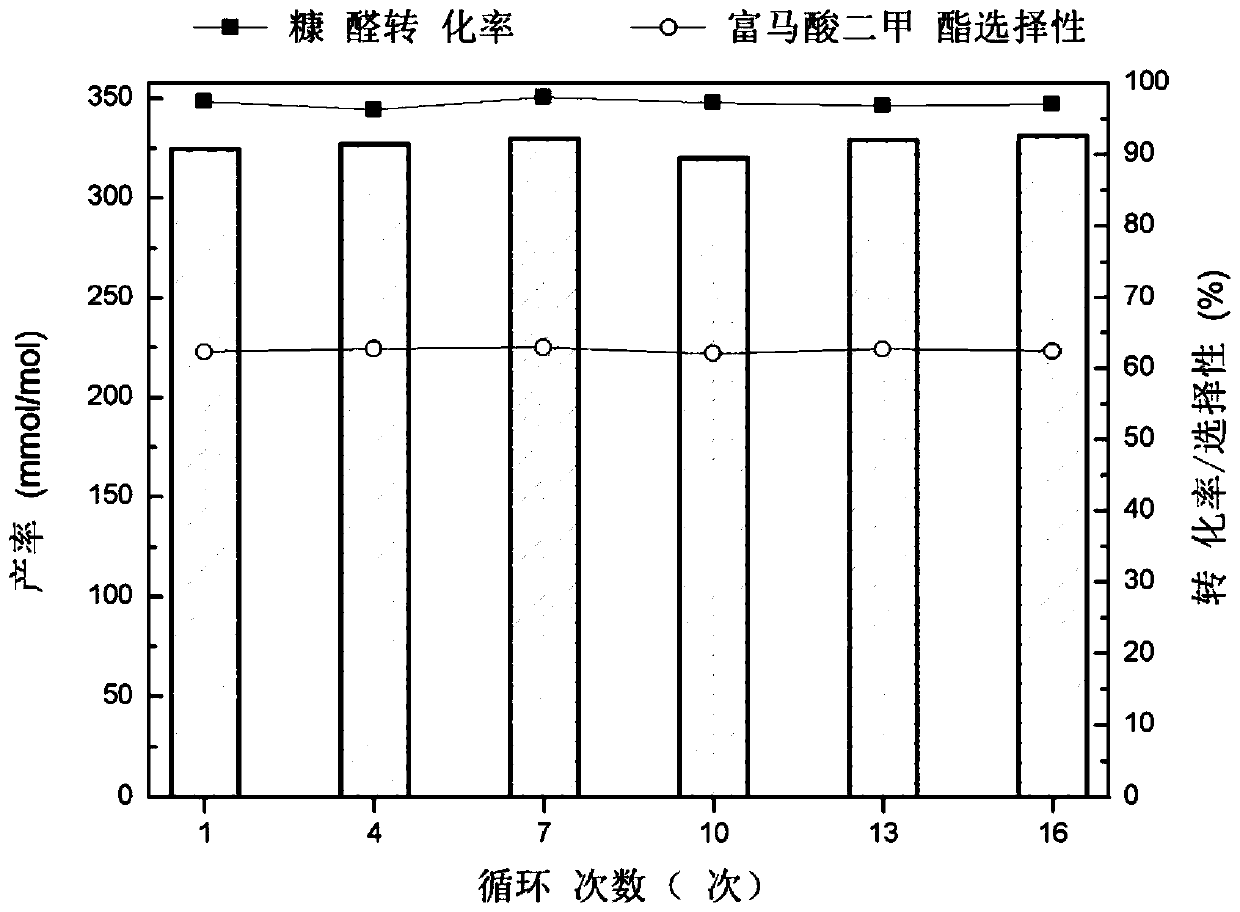
A method for selective catalytic oxidation of biomass-based furan compounds
A technology for catalytic oxidation of furan, which is applied in the preparation of organic compounds, catalysts for physical/chemical processes, catalysts for organic compounds/hydrides/coordination complexes, etc., and can solve complex, impractical, and costly production and purification processes. High cost and other problems, achieve the effect of intermittent and continuous production, simple process and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1. Ionic liquid 1‐(4‐sulfonic acid butyl)‐3‐methylimidazolium phosphomolybdenum divanadate copper salt (BSmimCuH 2 PMo 10 V 2 o 40 ) preparation
[0028] (1) Weigh an equimolar amount of methylimidazole and butane sultone to react at 30°C for 18h; the product obtained after the reaction is washed with ether and dried under vacuum at 50°C to obtain a white solid inner salt 1-methyl- 3‐butanesulfonate imidazole;
[0029] (2) Phosphomolybdovanadium heteropolyacid H 5 PMo 10 V 2 o 40 Dissolve in deionized water, stir until completely dissolved; weigh the corresponding molar amount of basic copper carbonate according to the ratio of basic copper carbonate: heteropoly acid molar mass of 1:2, and slowly add it to phosphomolybdenum divanadium heteropoly acid h 5 PMo 10 V 2 o 40 solution, stirred at room temperature for 18h, then removed the solvent with a rotary evaporator at 70°C, and dried in vacuum at 50°C for 48h to obtain the heteropolyacid salt CuH 3 PMo 10 ...
Embodiment 2
[0037] The difference between this embodiment and embodiment 1 is:
[0038] 1. Ionic liquid 1‐(4‐sulfonic acid butyl)‐3‐methylimidazolium phosphomolybdate BSmimH 2 PMo 12 o 40 Preparation of:
[0039] (1) Weigh an equimolar amount of methylimidazole and butane sultone to react at 45°C for 24 hours; after the reaction, wash with ether and dry under vacuum at 65°C to obtain the white solid inner salt 1-methyl-3- Butanesulfonate imidazole;
[0040] (2) Weigh the heteropolyacid H of equimolar amount 3 PMo 12 o 40 And the internal salt 1‐methyl‐3‐butanesulfonic imidazole prepared above, the heteropolyacid H 3 PMo 12 o 40 After dissolving the inner salt and the inner salt respectively with deionized water, the inner salt solution was added dropwise to the heteropoly acid H while stirring. 3 PMo 12 o 40 In the solution, react at room temperature for 48h;
[0041] (3) After the reaction, the solvent was removed by rotary evaporation to obtain a yellow solid, which was vac...
Embodiment 3
[0046] The difference between this embodiment and embodiment 1 is:
[0047] 1. Ionic liquid 1‐(4‐sulfonic acid butyl)‐3‐methylimidazolium phosphomolybdate copper salt (BSmimCuPMo 12 o 40 ) preparation
[0048] (1) Weigh an equimolar amount of methylimidazole and butane sultone to react at 30°C for 18 hours; after the reaction, wash with ether and dry under vacuum at 50°C to obtain a white solid internal salt 1-methyl-3- Butanesulfonate imidazole;
[0049] (2) phosphomolybdic acid is dissolved in deionized water, stirred until phosphomolybdic acid is completely dissolved; by basic copper carbonate: the molar mass of phosphomolybdic acid is the ratio of 1:2 to take the basic copper carbonate of corresponding molar quantity, Slowly added to the phosphomolybdic acid solution, stirred at room temperature for 18 hours, then removed the solvent with a rotary evaporator at 70°C, and dried in vacuum at 50°C for 48 hours to obtain the heteropoly salt CuHPMo 12 o 40 ;
[0050] (3) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com