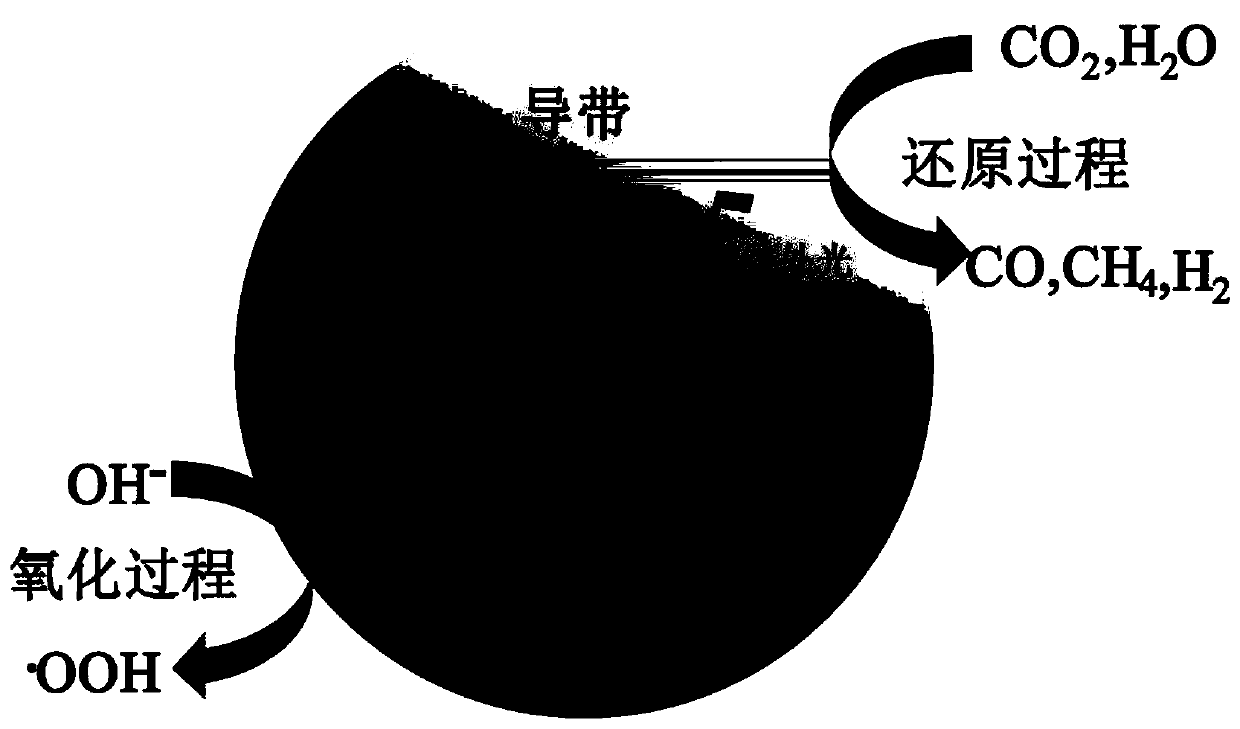
Hydroxyl-modified oxygen-vacancy strontium titanate photocatalytic material and its preparation and application
A catalytic material, the technology of strontium titanate, applied in the application of photoreduction of carbon dioxide, the field of preparation of strontium titanate photocatalytic materials, can solve the problem that strontium titanate can only use ultraviolet light, etc., to reduce the thermodynamic barrier and stabilize Enhanced performance and improved photocatalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
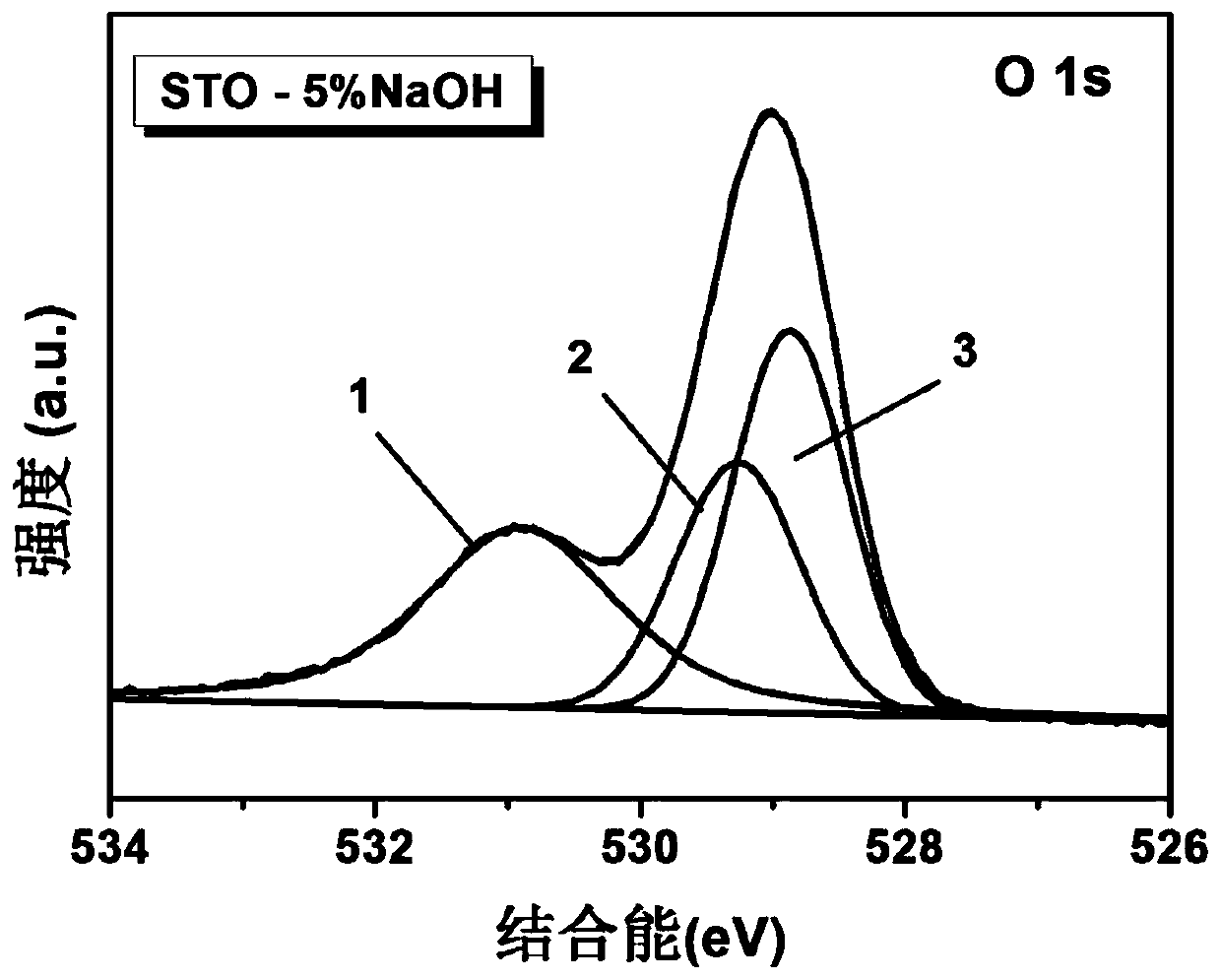
[0030] Preparation of hydroxyl-modified strontium titanate containing oxygen vacancies: Weigh 0.3g of commercial strontium titanate and 0.1g of sodium borohydride, grind them evenly, transfer them to a tube furnace for sintering at 300°C with argon for one hour; centrifuge the sintered samples Separation, washing with deionized water several times to remove residual sodium borohydride, and drying to obtain a sample containing oxygen vacancies, labeled as STO-NaBH 4 ;Weigh 0.1g STO-NaBH 4 Add 10ml of deionized water and ultrasonicate for half an hour to obtain evenly dispersed colloidal strontium titanate; weigh 0.1g of sodium hydroxide, add 10ml of deionized water to dissolve, and prepare a standard solution of sodium hydroxide. Use a pipette gun to pipette 5% sodium hydroxide solution into the strontium titanate colloid, sonicate for a few seconds, then transfer the obtained mixture to a vacuum drying oven at 60°C for 24 hours, and grind to obtain the final sample.
[0031] ...
Embodiment 2
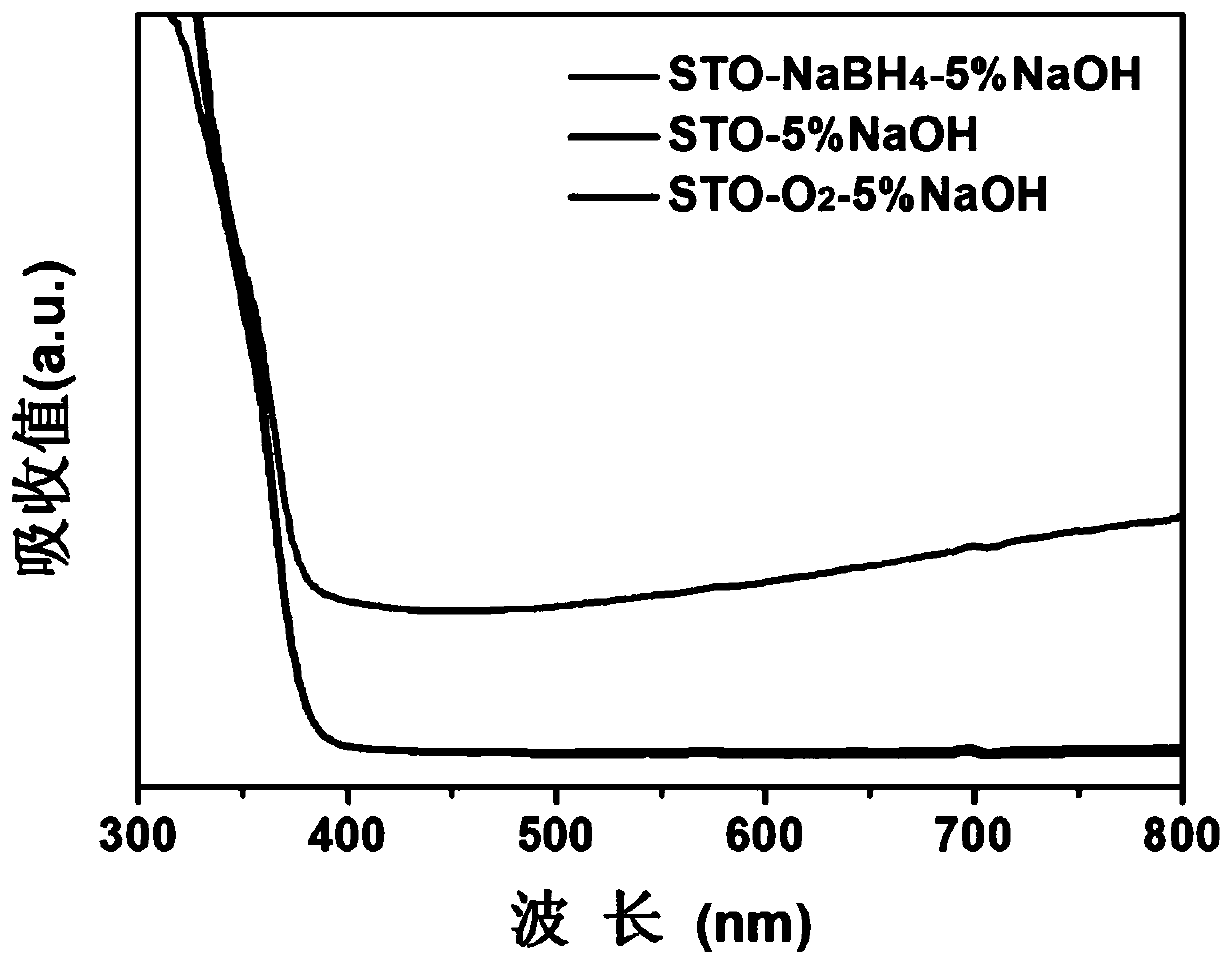
[0036] Effect of the amount of alkali metal hydroxide on the product: Weigh five parts of 0.1g oxygen-vacancy strontium titanate samples (STO-NaBH 4 , synthesized according to Example 1), add 10ml of deionized water, ultrasonic for half an hour, uniformly disperse, and then add mass fractions of 0%, 1%, 3%, 5%, and 7% respectively in five samples Sodium hydroxide, after a few seconds of ultrasonication, put it into a vacuum drying oven at 60°C for 24 hours in a vacuum, and grind to obtain the final product. Through the structural analysis of the product in this case and the evaluation of photoreduction carbon dioxide activity, it can be seen that when the mass fraction of sodium hydroxide is 5%, the photoreduction activity of the product is optimal, which can be attributed to the number of hydroxyl groups reaching a better value.
Embodiment 3
[0038] Influence of the type of alkali metal hydroxide on the product: Weigh three 0.1g samples of strontium titanate containing oxygen vacancies (STO-NaBH 4 , synthesized according to Example 1), add 10ml of deionized water for ultrasonic dispersion, then add lithium hydroxide, sodium hydroxide, and potassium hydroxide with a mass fraction of 5% respectively, and put them into a vacuum drying oven at 60°C under vacuum after ultrasonic Dry for 24 hours and grind to get the final product. According to the structural analysis of the product in this case and the evaluation of photoreduction carbon dioxide activity, it can be known that the reduction performance of the sample is the best when the alkali metal hydroxide used is sodium hydroxide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com