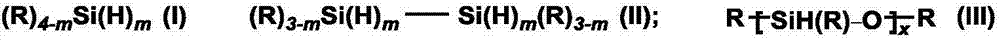Method for preparing deuterated aromatic organic compound
A technology of aromatic compounds and compounds, applied in the preparation of organic compounds, organic chemical methods, and hydrocarbon production from halogen-containing organic compounds, etc., can solve the problems of harsh reaction conditions and poor compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Research on alkali metal salts and deuterated solvents using 5-bromo-N-methylindole as a standard substrate dehalogenation reaction:
[0043]
[0044]
[0045] Among them, MA represents alkali metal salt, [CH 3 (H)SiO] n Represents polymethylhydrogensiloxane, equiv refers to the equivalent, deuterated solvent represents a deuterated reagent or a mixture of a deuterated reagent and a conventional solvent, and the volume is 1 mL. Among them, CD 3 CN is deuterated acetonitrile, THF is tetrahydrofuran, Et 2 O is diethyl ether, DME is dimethylethylene ether, MTBE is methyl tert-butyl ether, DMSO-d 6 is deuterated dimethyl sulfoxide. When using a mixed solvent, the molar ratio of the deuterated reagent to the raw material should be ≥1:1, preferably 5:1.
Embodiment 2
[0046] Embodiment 2: Preparation of 5-deuterium-N-methylindole and its gram-level reaction
[0047]
[0048] Dissolve 105 mg of 5-bromo-N-methylindole (0.5 mmol) and 70.15 mg of potassium methoxide (1 mmol) in 1 mL of deuterated acetonitrile, add 200 μL of hexamethyldisilane (1 mmol) dropwise to the mixture, and stir at room temperature Reaction 3h. After the reaction was complete, add 10 mL of water to quench, extract with 30 mL of diethyl ether three times, collect the organic phase, and remove the solvent under reduced pressure. Separation and purification by column chromatography, the eluent is petroleum ether: ethyl acetate = 20:1 (v / v), to obtain 57 mg of 5-deuterium-N-methylindole (pale yellow liquid, yield 87%). 1 H NMR (400MHz, CDCl3) δ=7.72(s,1H),7.43–7.36(m,1H),7.32(s,1H),7.14–7.08(m,1H),6.57(d,J=2.9Hz, 1H), 3.83(s, 3H). GC-MS (EI+): 132.1.
[0049] 2.1 g of 5-bromo-N-methylindole (10 mmol) and 1.403 g of potassium methylate (20 mmol) were dissolved in a mixe...
Embodiment 3
[0050] Embodiment 3: the preparation of 5-deuterium-N-methylindole under mixed solvent
[0051]
[0052] Dissolve 105 mg of 5-bromo-N-methylindole (0.5 mmol) and 70.15 mg of potassium methoxide (1 mmol) in a mixture of 130 μL of deuterated acetonitrile (2.5 mmol) and 870 μL of tetrahydrofuran, and add 200 μL of hexamethanol dropwise to the mixture Base disilane (1mmol), stirred at room temperature for 3h. After the reaction was complete, add 10 mL of water to quench, extract with 30 mL of diethyl ether three times, collect the organic phase, and remove the solvent under reduced pressure. Separation and purification by column chromatography, the eluent is petroleum ether: ethyl acetate = 20:1 (v / v), to obtain 48 mg of 5-deuterium-N-methylindole (pale yellow liquid, yield 73%). 1 H NMR (400MHz, CDCl3) δ=7.72(s,1H),7.43–7.36(m,1H),7.32(s,1H),7.14–7.08(m,1H),6.57(d,J=2.9Hz, 1H), 3.83(s, 3H). GC-MS (EI+): 132.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com