High-solid and low-viscosity organosilicone-modified acrylate resin, preparation method and applications
An acrylic resin, high-solid and low-viscosity technology, used in biocide-containing paints, coatings, antifouling/underwater coatings, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] The formula of the preparation method of the organosilicon macromonomer G with a hydroxyhydrocarbyl group in the side chain and a methacrylic acid group in a single end is as follows:
[0106]
[0107] Hydroxyl protection: In a 1L reactor, slowly drop 338.1g (2.1mol) of hexamethyldisilazane into 232g (4mol) of allyl alcohol at room temperature. After the dropwise addition, the temperature of the reaction system was raised to 100° C., and the reaction was continued at this temperature for 6 hours, and then the reaction was stopped. The fraction at 98-100° C. was collected under normal pressure to obtain 473.2 g of allyloxytrimethylsilane with a yield of 91%.
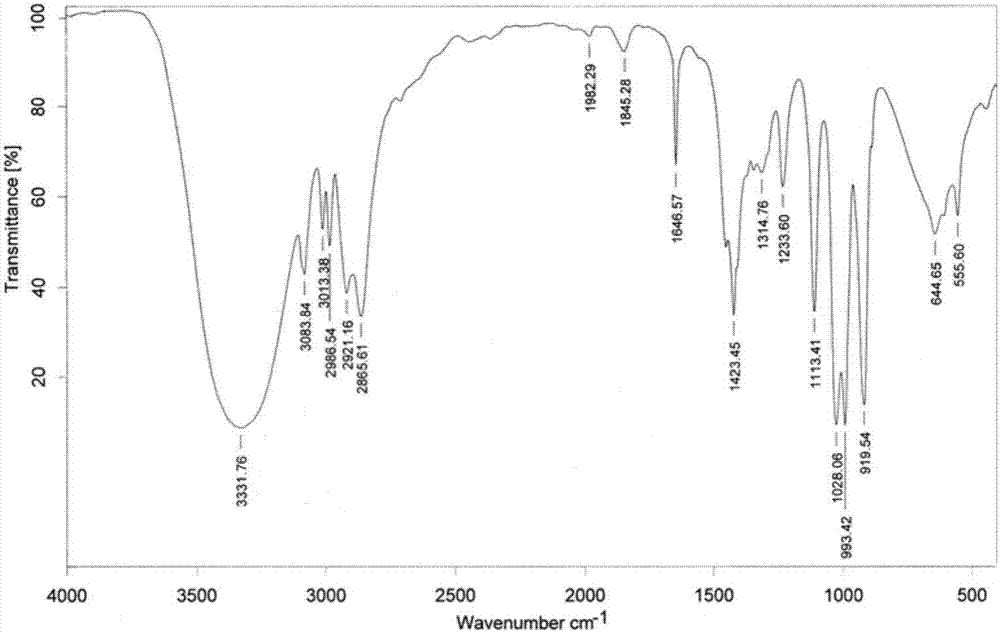
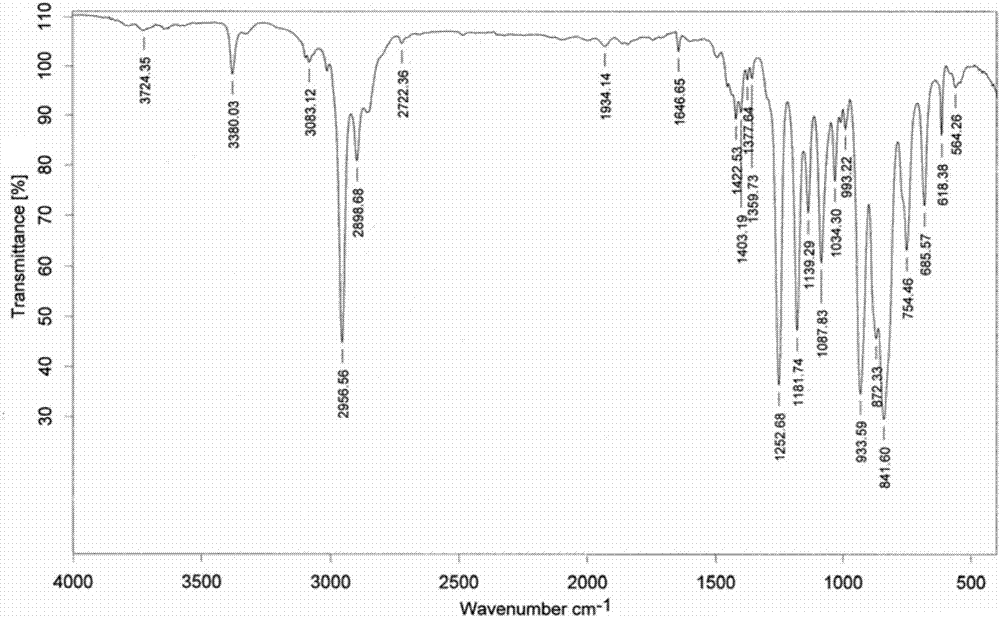
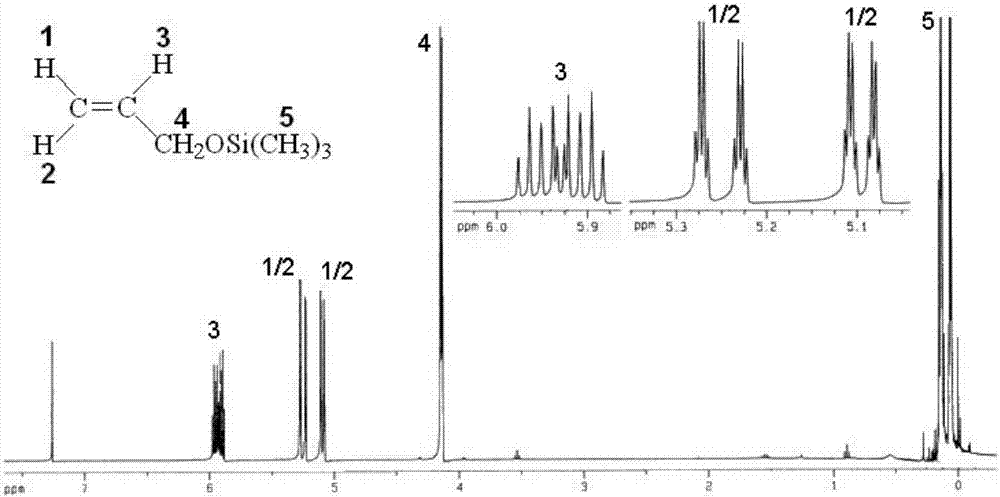
[0108] like Figure 1~3 as shown, figure 1 It is the infrared spectrogram of allyl alcohol. In the figure: the ordinate Transmittance refers to the transmittance, and the abscissa wavenumbers refers to the wave number. Allyl alcohol is at 3332cm -1 There are broad hydroxyl stretching vibration absorption peak...
Embodiment 2
[0116] Hydroxyl protection: In a 1L reactor, slowly drop 338.1g (2.1mol) of hexamethyldisilazane into 288g (4mol) of 3-buten-1-ol at room temperature. After the dropwise addition, the temperature of the reaction system was raised to 100° C., and the reaction was continued at this temperature for 6 hours, and then the reaction was stopped. The fraction at 110-115°C was collected under normal pressure to obtain 558 g of butoxytrimethylsilane with a yield of 96.9%.
[0117] One-time hydrosilylation: In a 1L reaction kettle, add 436.3g (3.03mol) butyloxytrimethylsilane and 0.5g chloroplatinic acid catalyst in sequence, and after passing nitrogen gas for 20min, raise the temperature of the reaction system to 100°C At this temperature, 180 g (1 mol) of trimethylcyclotrisiloxane was added dropwise, and the reaction was terminated after 8 hours of reaction. Low boilers were distilled off under reduced pressure to obtain 601 g of trimethylsiloxyalkyl-modified cyclotrisiloxane with a y...
Embodiment 3
[0122] The allyl alcohol in the hydroxyl protection reaction of Example 1 is replaced by 4-penten-1-alcohol, the amount of n-butyllithium in the anionic polymerization is changed from 1mol to 0.5mol, and the substance of dimethyl monochlorosilane The amount of is changed from 1.1mol to 0.55mol, and other reaction conditions are as described in Example 1, and the molecular weight and structure are different from the organosilicon macromer of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydroxyl value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com