Method for preparing isradipine impurity III
A technology for isradipine and impurities, which is applied in the field of preparation of isradipine impurity III, can solve problems such as separation difficulties and affecting the quality of the final product isradipine, and achieve the effects of easy-to-obtain raw materials, short routes, and simple preparation processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
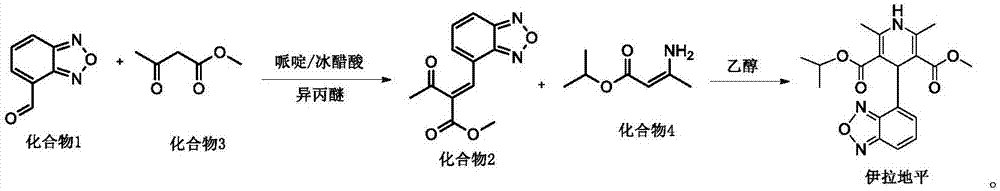
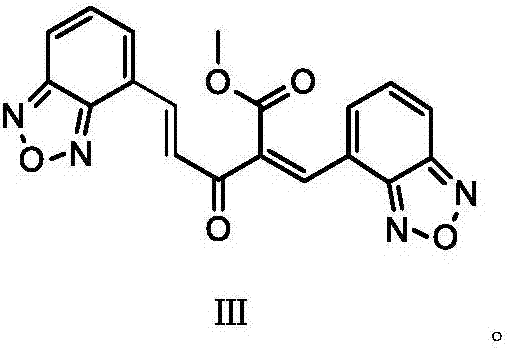
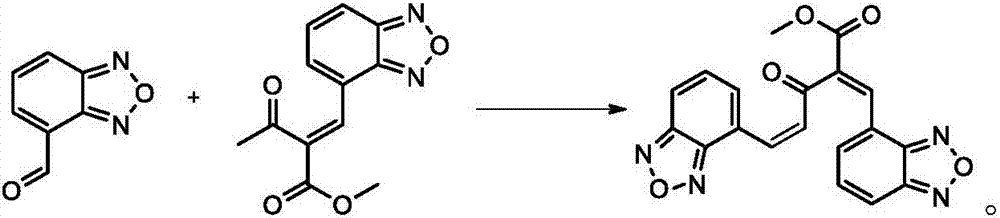
[0024] The invention provides a preparation method of isradipine impurity III, which is characterized in that: benzoxadiazole-4-carbaldehyde and 2-acetyl-3-benzofurazan-4-yl-methyl acrylate are used as The raw material undergoes a condensation reaction under weakly alkaline and organic conditions, and is separated to obtain isradipine impurity III. The reaction formula is as follows:
[0025]
[0026] Further, the preparation method of the isradipine impurity III specifically includes the following steps:
[0027] S1. Add 5-10g of 2-acetyl-3-benzofurazan-4-yl-methyl acrylate to 100-180ml of organic solvent and stir. After fully dissolving, add benzoxadiazole-4-formaldehyde 5- 7g, 0.3-1.1g of glacial acetic acid and 0.1-0.5g of catalyst, carry out heating and reflux reaction;
[0028] S2, filtering the reaction solution obtained in step S1 to obtain a filtrate;
[0029] S3. Add 10-30ml of organic solvent to the filtrate obtained in step S2, heat to reflux for 1-2h, cool do...
Embodiment 1
[0037] Add 130mL of isopropyl ether and 8.31g of compound 2 to the reaction flask in turn, stir and dissolve, then add 5g of compound 1, 0.6g of glacial acetic acid and 0.2g of piperidine, heat up and reflux for 4h; filter the reaction solution to obtain the filtrate, and pour the filtrate 15 mL of ethyl acetate was added to the mixture, stirred and heated to reflux for 1 h, cooled and filtered, and dried at 60°C to obtain isradipine impurity III with a purity of 99.5% and a weight of 6.75 g.
[0038] The mass spectrometry data and NMR data detection of the product obtained in this embodiment are as follows: ESI-MS: 377.30 [M+H] + ;1H-NMR: (δ-DMSO), δ3.814(3H, s), δ7.515~7.542(1H,d), δ7.578~7.627(2H,m), δ7.831~7.858(1H, d), δ7.905~7.916(1H,d), δ7.986~7.995(1H,d), δ8.041~8.079(1H,m), δ8.079~8.091(1H,d), δ8.229 (1H,s).
Embodiment 2
[0040] Add 100mL tetrahydrofuran and 5g compound 2 to the reaction flask in sequence, stir and dissolve, then add 5g compound 1, 0.4g glacial acetic acid and 0.1g piperidine, heat and reflux for 2h; filter the reaction solution to obtain the filtrate, add 10mL to the filtrate Acetone, stirred and heated to reflux for 2 hours, cooled and filtered, and dried at 60°C to obtain isradipine impurity III with a purity of 98.9% and a weight of 5.81g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com