Method for hydrogenation and arsenic removal of distillate oil
A technology for distillate oil and arsenic removal, which is applied in chemical instruments and methods, processing of hydrocarbon oil, petroleum industry, etc., can solve the problems of complex composition of liquid naphtha hydrocarbons, poor hydrodearsenic activity, and poor resistance to impurities, etc. To achieve the effect of good hydrodearsenic activity and stability, low price and mild process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] First, 8.0 g of the water-soluble chitosan pore-enlarging agent was added to deionized water at 50° C., and then acetic acid was added dropwise until the chitosan was completely dissolved to obtain an acid solution containing the pore-enlarging agent. Weigh 1.46g of phosphoric acid and 7.35g of magnesium nitrate respectively, and completely dissolve phosphoric acid and magnesium nitrate in 70g of distilled water to form an aqueous solution containing phosphorus and magnesium. Weigh 350g of pseudo-boehmite powder and 20.0g of fenugreek powder into the kneader, mix well, then add the mixed solution of phosphoric acid and magnesium nitrate, and finally add the acid solution containing chitosan to the pseudo-boehmite The powder is evenly kneaded, and then kneaded-extruded into a clover shape. Dry at 120° C. for 8 hours, and calcined at 700° C. for 4 hours to obtain an alumina carrier 1 containing phosphorus and magnesium. In carrier 1, phosphorus pentoxide is 0.5wt%, and m...
Embodiment 2
[0039] Add 8.0 g of the water-soluble chitosan pore-enlarging agent into deionized water at 50° C., and then add acetic acid dropwise until the chitosan is completely dissolved to obtain an acid solution containing the pore-enlarging agent. Weigh 1.09g of phosphoric acid and 9.12g of magnesium nitrate respectively, completely dissolve phosphoric acid and magnesium nitrate in 70g of distilled water to form an aqueous solution containing phosphorus and magnesium. Weigh 350g of pseudo-boehmite powder and 20.0g of fennel powder into the kneader, mix well, then add the mixed solution of phosphoric acid and magnesium nitrate, and finally add the acid solution containing chitosan to the pseudo-boehmite The stone powder is evenly kneaded, and then kneaded-extruded into a clover shape. Dry at 120° C. for 8 hours, and calcined at 700° C. for 4 hours to obtain an alumina carrier 1 containing phosphorus and magnesium. Then use phosphorus and magnesium to modify the surface of the carrier...
Embodiment 3
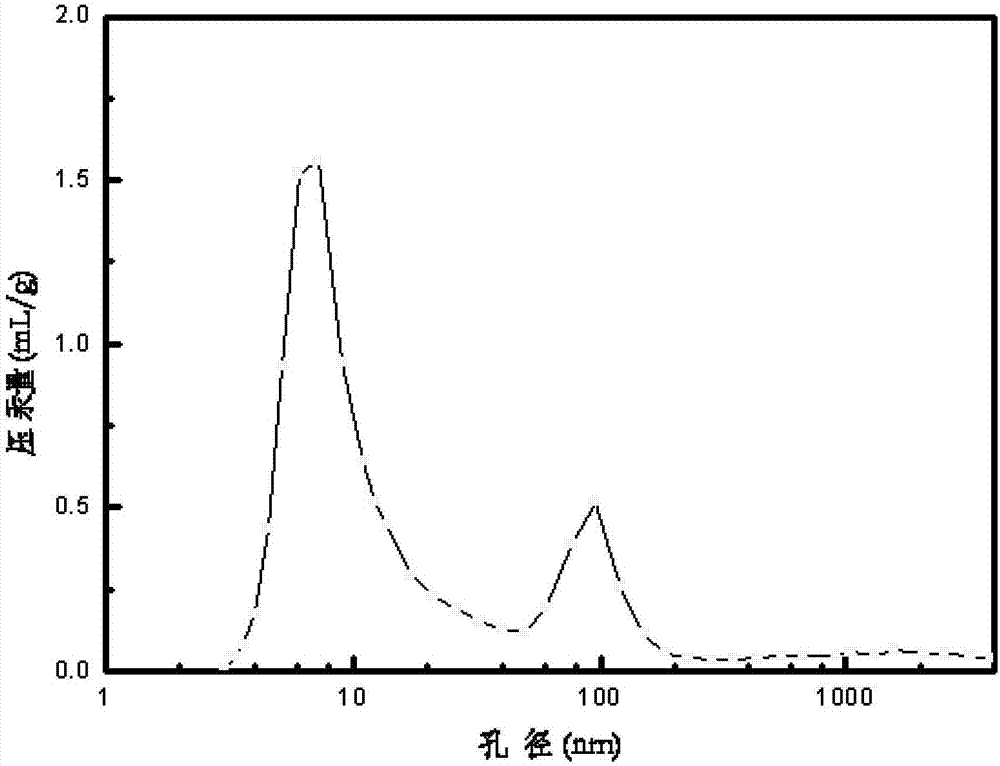
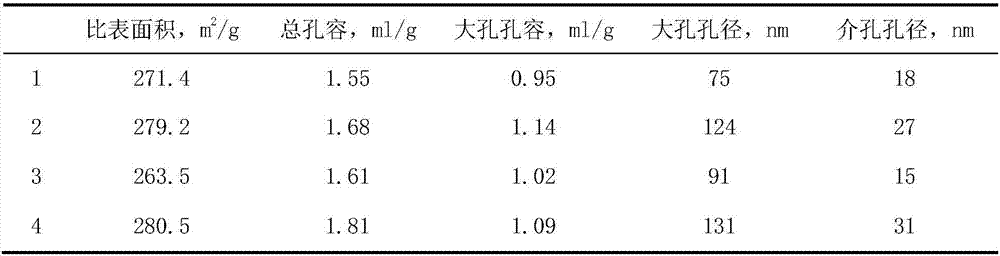
[0044] The preparation method of the carrier was carried out according to Example 1. The difference is that the water-soluble chitosan pore-enlarging agent is replaced by a non-water-soluble chitosan pore-enlarging agent, and the chitosan formic acid solution is stirred for 30 minutes with a magnetic stirrer. An alumina support 3 with a macroporous structure was obtained. The content of the auxiliary components phosphorus and magnesium in the carrier accounts for the percentage of the carrier mass, respectively P 2 o 5 1.8wt%, MgO2.0wt%. Its specific surface area and pore size distribution are shown in Table 1.
[0045] Nickel nitrate and ammonium molybdate were prepared as an impregnating solution, and 100 g of a carrier containing macroporous alumina was impregnated. The catalyst was dried at 130° C. for 6 hours and then calcined at 650° C. for 6.0 hours to obtain Hydrodearsenization Catalyst 3 . Catalyst 3 mainly consists of 9.0 wt% nickel oxide, 4.0 wt% molybdenum oxi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size distribution | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com