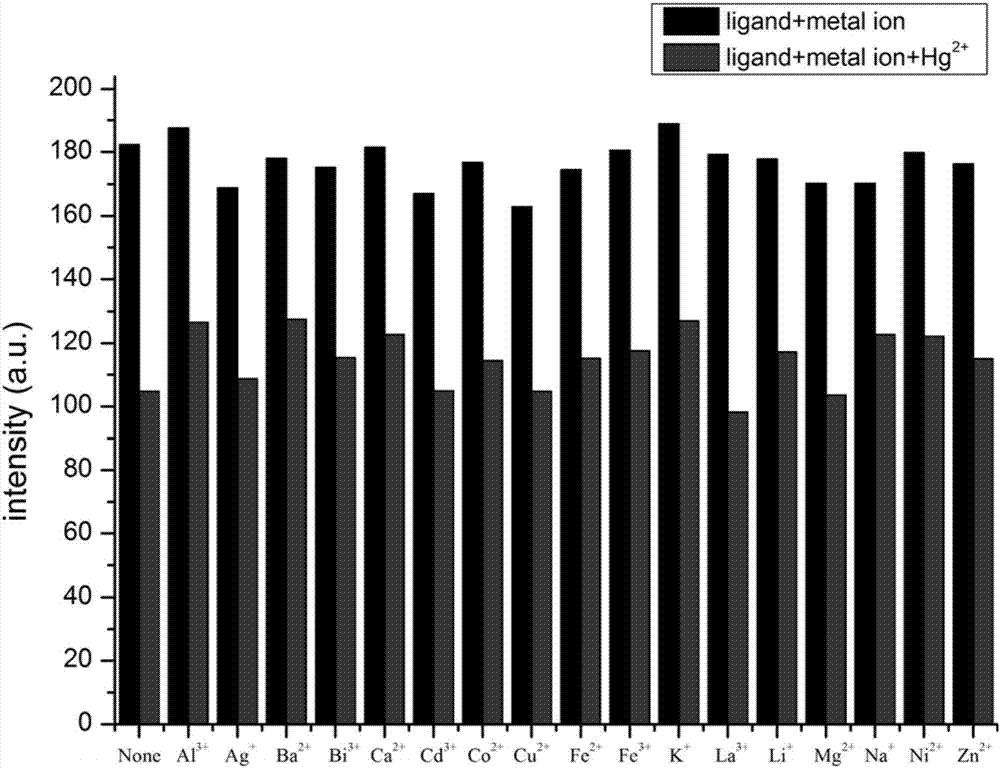
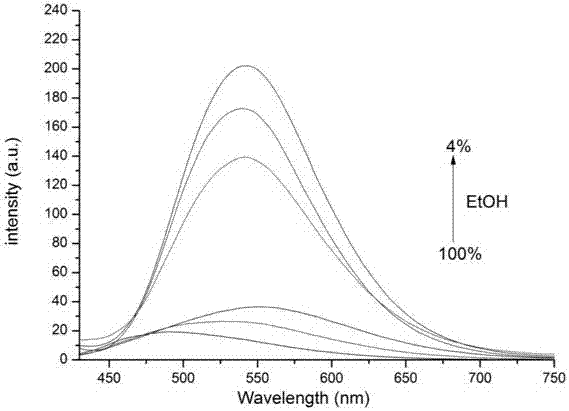
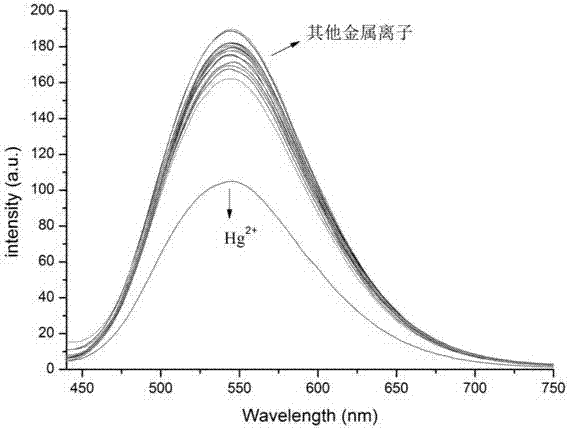
Tetraphenyl ethylene-diaminomaleonitrile derivative and preparation method and application thereof
A technology of diaminomaleonitrile and tetraphenylethylene, which is applied in the field of tetraphenylethylene-diaminomaleonitrile derivatives and its preparation, can solve the problems of long response time of mercury ions, low detection sensitivity, and poor water solubility, and achieve The preparation method is simple and easy, the effect of high luminous intensity and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043]The invention provides a kind of preparation method of tetraphenylethylene-diaminomaleonitrile derivative, comprising the following steps:
[0044] A) under the action of a catalyst, after reacting triphenylbromoethylene, 4-formylphenylboronic acid, potassium carbonate and the first solvent, a reaction intermediate is obtained;
[0045] B) reacting the reaction intermediate obtained in the above steps, diaminomaleonitrile and the second solvent again to obtain tetraphenylethylene-diaminomaleonitrile derivatives.
[0046] In the present invention, under the action of a catalyst, triphenylbromoethylene, 4-formylphenylboronic acid, potassium carbonate and a first solvent are reacted to obtain a reaction intermediate.
[0047] The present invention has no special restrictions on the selection of the 4-formylphenylboronic acid, and the conventional 4-formylphenylboronic acid well-known to those skilled in the art can be selected. Those skilled in the art can select according ...
Embodiment 1
[0074]Dissolve 6mmol of triphenylbromoethylene and 8mmol of 4-formylphenylboronic acid in 50ml of methanol and 50ml of toluene and mix, add 3.5g of calcium carbonate and 0.05g of bis(triphenylphosphine)palladium dichloride, in a nitrogen atmosphere , heated and stirred at 75°C, condensed and refluxed for 16 hours, and cooled to room temperature. Purified by column chromatography, the eluent was n-hexane / dichloromethane, and a yellow-green solid was isolated, which was the first product I (reaction intermediate).
[0075] 2 mmol of the first product I and 2 mmol of diaminomaleonitrile were dissolved in 35 ml of ethanol, heated and stirred at 80° C., condensed and refluxed for 23 hours, and cooled to room temperature. Purified by column chromatography, the eluent was n-hexane / dichloromethane, and a yellow solid was obtained, which was the ligand HL (tetraphenylethylene-diaminomaleonitrile derivative).
[0076] The ligand HL prepared in Example 1 of the present invention was cha...
Embodiment 2
[0080] 6.6mmol of triphenylbromoethylene and 8.9mmol of 4-formylphenylboronic acid were dissolved in 50ml of methanol and 50ml of toluene and mixed, adding 3.5g of calcium carbonate and 0.05g of two (triphenylphosphine) palladium dichloride, in Under nitrogen atmosphere, heat and stir at 75°C, condense and reflux for 16 hours, and cool to room temperature. Purified by column chromatography, the eluent was n-hexane / dichloromethane, and a yellow-green solid was isolated, which was the first product I.
[0081] Dissolve 2mmol of the first product I and 2mmol of diaminomaleonitrile in 20ml of ethanol, heat and stir at 90°C, reflux for 20 hours, and cool to room temperature. Purified by column chromatography, the eluent was petroleum ether / ethyl acetate, and a yellow solid was obtained, which was the ligand HL.
[0082] The ligand HL prepared in Example 1 of the present invention was characterized and tested.
[0083] Ligand HL was characterized and identified as C 31 h 22 N 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com