Recombinant carbonyl reductase mutant, gene, vector, engineering bacterium and application of recombinant carbonyl reductase mutant
A recombinant vector and mutant technology, applied in genetic engineering, oxidoreductase, applications, etc., can solve the problems of difficult to meet the requirements of optical purity of products, insufficient diastereomeric induction, low yield, etc., and achieve catalytic activity and bottom. Improved chemical tolerance, shortened reaction time, and reduced reaction costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
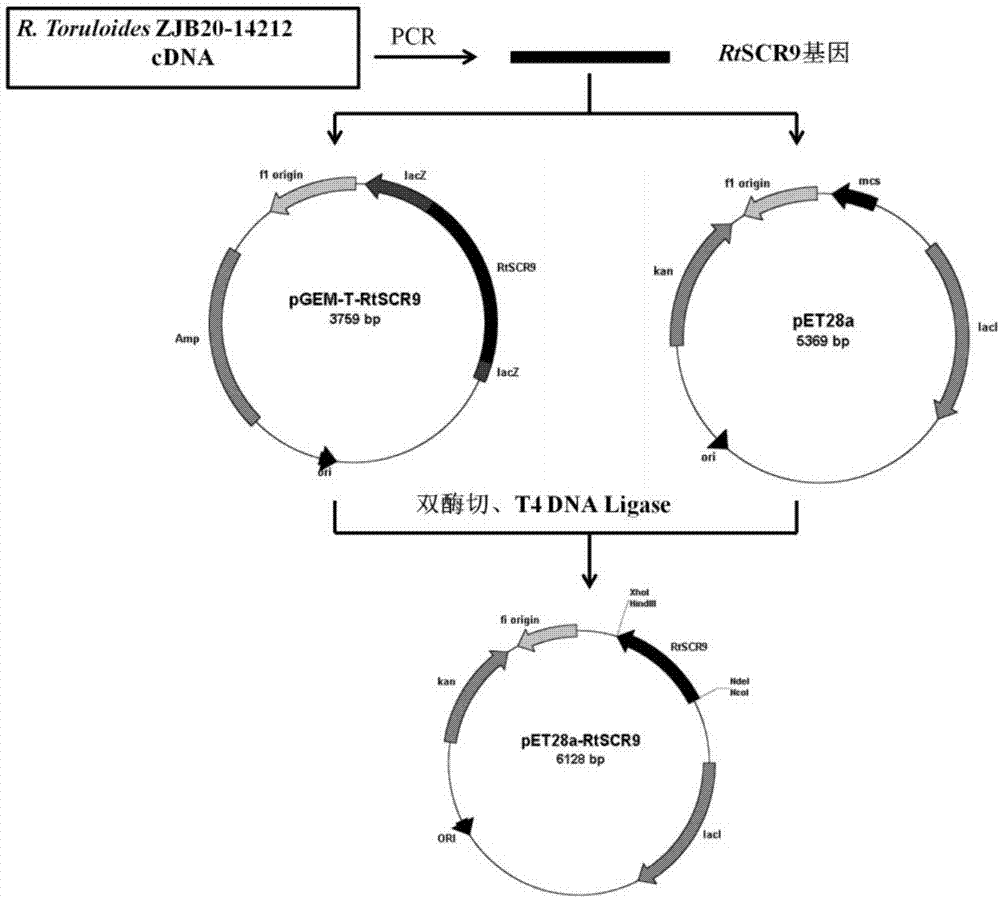
[0038] Example 1: Construction of recombinant carbonyl reductase genetically engineered bacteria BL21(DE3) / pET28a-RtSCR9
[0039] From Rhodosporidium toruloides (Rhodosporidium toruloides) ZJB2014212 (CCTCC NO.M2014613, disclosed in the patent application CN 105039361 A) has catalytic (S)-6-chloro-5-hydroxyl-3-oxohexanoic acid tertiary The enzyme capable of generating (3R,5S)-6-chloro-3,5-dihydroxyhexanoic acid tert-butyl ester from butyl ester is the carbonyl reductase RtSCR9 involved in the present invention.
[0040] The total mRNA of Rhodosporidium toruloides) ZJB2014212 cells was extracted by TRIzol reagent of Ambion Company. Using 1 mg of mRNA as a template, it was reverse-transcribed to synthesize cDNA using the ReverTra AceqPCR RT Kit. Using the cDNA as a template, PCR amplification was performed under the action of primer 1 (ATGTCTTCGCCTACTCCCAACGTC) and primer 2 (CTACCATGGCAAGAACGTCCCGTC). PCR reaction conditions: pre-denaturation at 95°C for 5min, 95°C for 30s, 65...
Embodiment 2
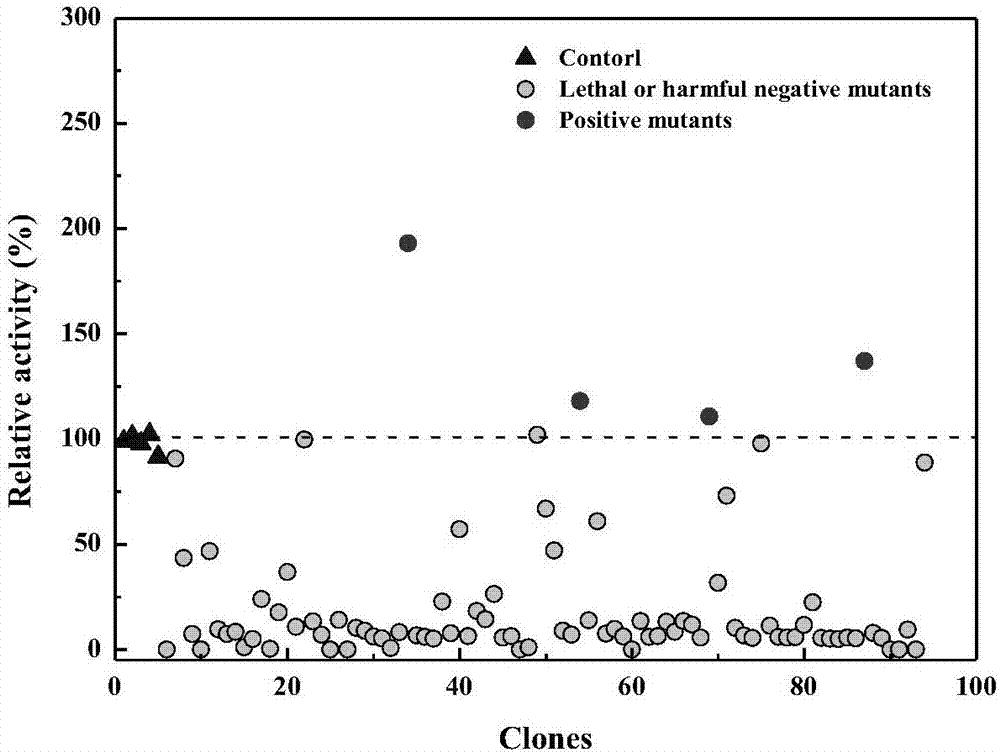
[0041] Embodiment 2: Obtaining of recombinant carbonyl reductase mutant
[0042] Using the recombinant bacteria (E.coli BL21(DE3) / pET28a-RtSCR9) containing the expression vector pET28a-RtSCR9 as the starting strain, through random mutation and site-directed saturation mutation techniques, the ability of carbonyl reductase to substrate (S)-6- Catalytic activity and substrate tolerance of tert-butyl chloro-5-hydroxy-3-oxohexanoate.
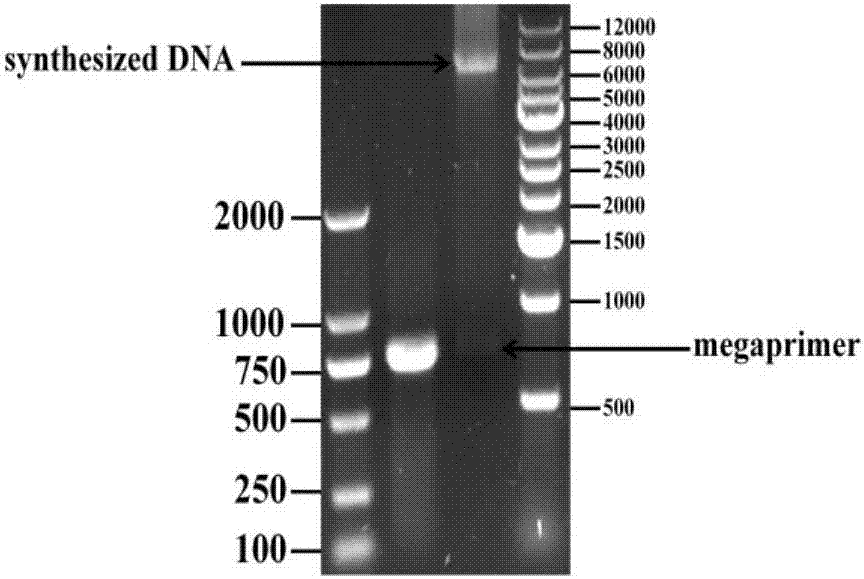
[0043] (1) Error-prone PCR and PCR with large primers
[0044] Error-prone PCR upstream primer 5: 5'-TATGTCTTCGCCTACTCCCAAC-3'
[0045] Error-prone PCR downstream primer 6: 5'-TCTACCATGGCAAGAACGTCC-3'
[0046] Error-prone PCR by changing the Mn in the PCR system 2+ , Mg 2+ The wrong bases are randomly incorporated into the amplified gene at a certain frequency, so as to obtain a randomly mutated DNA population. In the present invention, the plasmid DNA where the RtSCR9 gene (the nucleotide sequence is shown in SEQ ID NO.1 and the amino acid seque...
Embodiment 3
[0062] Example 3: Preparation of recombinant carbonyl reductase mutant wet thallus
[0063] Inoculate the recombinant Escherichia coli containing the recombinant carbonyl reductase mutant gene obtained in Example 2 into LB liquid medium containing kanamycin resistance at a final concentration of 50 μg / mL, culture at 37°C for 8 hours at 150 rpm, and then inoculate with 1 % inoculum size (v / v) was inoculated into fresh LB liquid medium containing a final concentration of 50 μg / mL kanamycin resistance, and cultured at 37°C and 150 rpm until the cell OD 600 After reaching 0.6-0.8, add IPTG with a final concentration of 0.1mM, induce culture at 28°C for 12h, centrifuge at 8000×g for 10min at 4°C, discard the supernatant, collect the precipitate, and obtain the mutant containing the recombinant carbonyl reductase Genetic recombinant E. coli wet cells. The wet thalline can be used directly as a biocatalyst or for protein purification. The wet cells of recombinant Escherichia coli (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com