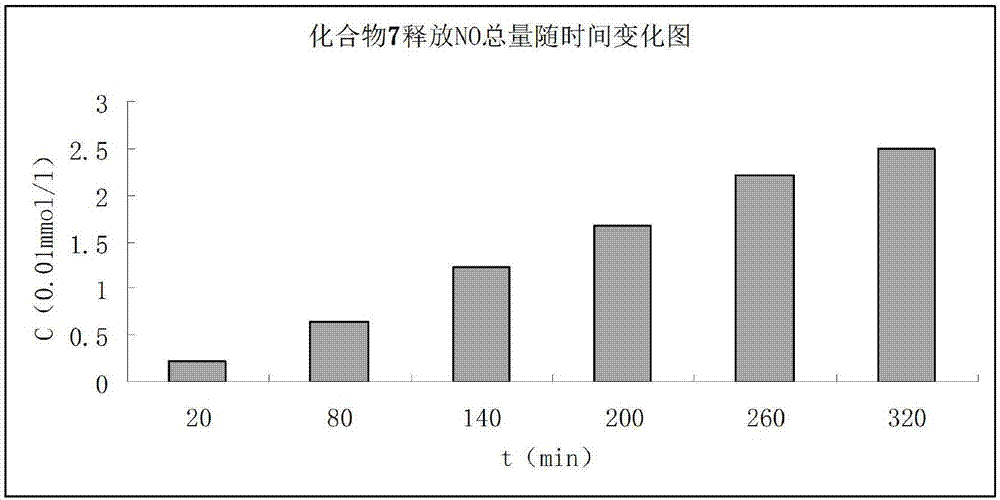
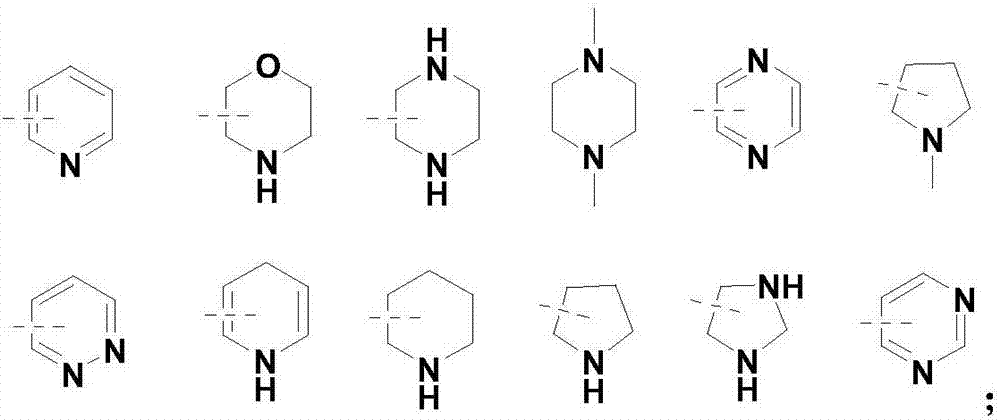
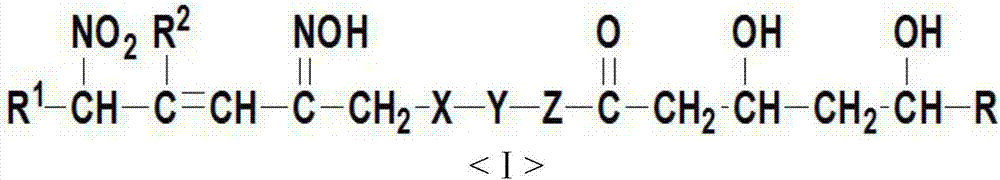FK409 NO donor type statins antilipemic medicine derivative and preparation method thereof
A technology of drugs and compounds, which is applied in the field of FK409 NO-donating statin blood lipid-lowering drug derivatives and its preparation, to achieve the effect of improving endothelial function, prolonging the action time, and increasing the half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Synthesis of (E,E)-4-ethyl-2,4-diene-hexanoic acid ethyl ester
[0069]
[0070] Ice bath, under nitrogen protection, in the anhydrous THF solution of 150ml, add the NaH (4.8g, 120mmol) of weight concentration of 60%, stir well, then add dropwise triethyl phosphoroacetate (20.16g, 90mmol) and ( E) A mixed solution of 2-ethyl-2-ene-butyraldehyde (5g, 51mmol) in anhydrous THF (20ml) was stirred for 15 minutes, then removed from the ice bath, warmed to room temperature and stirred for 4.5h. The reaction solution was poured into ice water, extracted with ethyl acetate, the organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, concentrated to obtain a crude product, and subjected to silica gel column chromatography (eluent: ethyl acetate / n-hexane=1 / 30) 5.0 g of a colorless oil were isolated.
Embodiment 2
[0072] Synthesis of (E,E)-4-ethyl-2,4-diene-1-hexanol
[0073]
[0074] At room temperature and protected from light, to the suspension of lithium aluminum hydride (0.742g, 19.52mmol) in anhydrous ether (100ml), the product obtained in Example 1 [(E,E)-4-ethyl- 2,4-diene-hexanoic acid ethyl ester (5.0g, 29.76mmol)] in ether solution, after stirring for 2 hours, TLC showed that the raw material still exists, add about 0.4g lithium aluminum hydride, stir for 3 hours and then process. The reaction was quenched with ethyl acetate, filtered, the organic layer was dried over anhydrous magnesium sulfate, and concentrated to obtain a crude product, which was subjected to silica gel column chromatography (eluent: ethyl acetate / cyclohexane=1 / 4) to obtain 2.71 g of colorless Transparent oil.
Embodiment 3
[0076] Synthesis of [(E,E)-4-ethyl-2,4-dien-1-yl]-4-chloro-butyric acid hexyl ester
[0077]
[0078] In an ice bath, add the product (E,E)-4-ethyl-2,4-diene-1-hexanol (2.87g, 22.78mol) obtained in Example 2 to triethylamine / dichloromethane (100ml / 150ml) into the mixed solution, add 4-chloro-butyryl chloride (6.8g, 0.0482mol) dropwise, stir and react under ice bath for 4h, extract the reaction solution with dichloromethane, wash the organic layer with saturated brine, and then wash with anhydrous magnesium sulfate Dry and concentrate to obtain a crude product, which is separated by silica gel column chromatography (eluent: ethyl acetate / cyclohexane=1 / 30) to obtain 4.3 g of a colorless transparent oil.
[0079] ESI-MS: 231.7(M+1) +
[0080] 1 H-NMR (400MHz, CDCl 3 , 25℃): 6.18(1H, d); 5.64(1H, m); 5.55(1H, q); 4.62(2H, d); 3.60(2H, t); 2.51(2H, t); , q); 2.10 (2H, m); 1.72 (3H, d); 0.99 (3H, t).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com