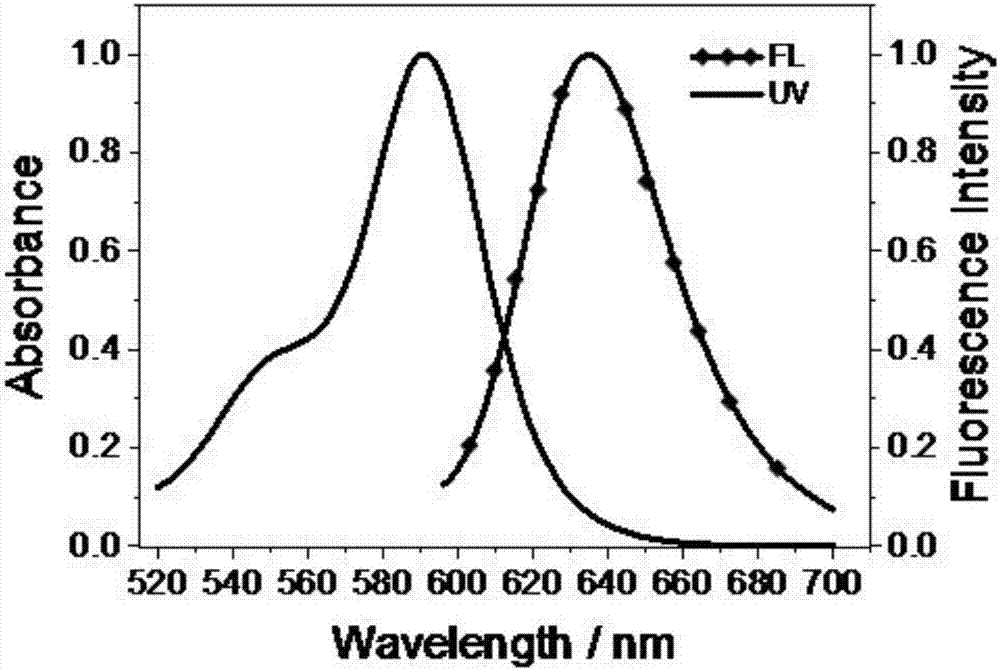
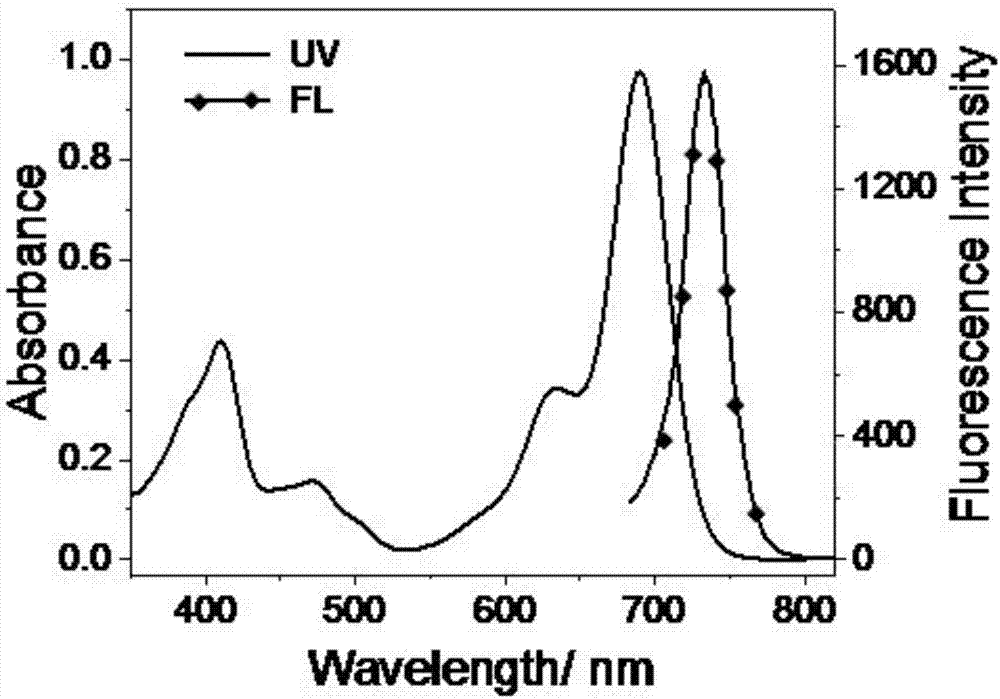
Lysosome-targeted fluorescent dye capable of realizing red emission and near-infrared emission, and preparation method and application thereof
A fluorescent dye and near-infrared technology, applied to luminescent materials, azo dyes, organic dyes, etc., can solve the problems of extended observation time, short excitation wavelength, short emission wavelength, etc., and achieve short dyeing time, good targeting, and The effect of high photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Weigh compound I and morpholine indole aldehyde in a three-necked flask, the molar ratio is 1:1.5, and add 30ml of anhydrous toluene as a solvent. Then, nitrogen gas was introduced, and piperidine and glacial acetic acid were added in sequence after the reaction was heated to 110°C. After the reaction mixture was refluxed under nitrogen atmosphere for 16 hours, the solvent was distilled off under reduced pressure, and the obtained residue was subjected to column chromatography (CH 2 Cl 2 / C2 h 5 OH=60 / 1) to obtain the target dye 1 as dark blue solid.
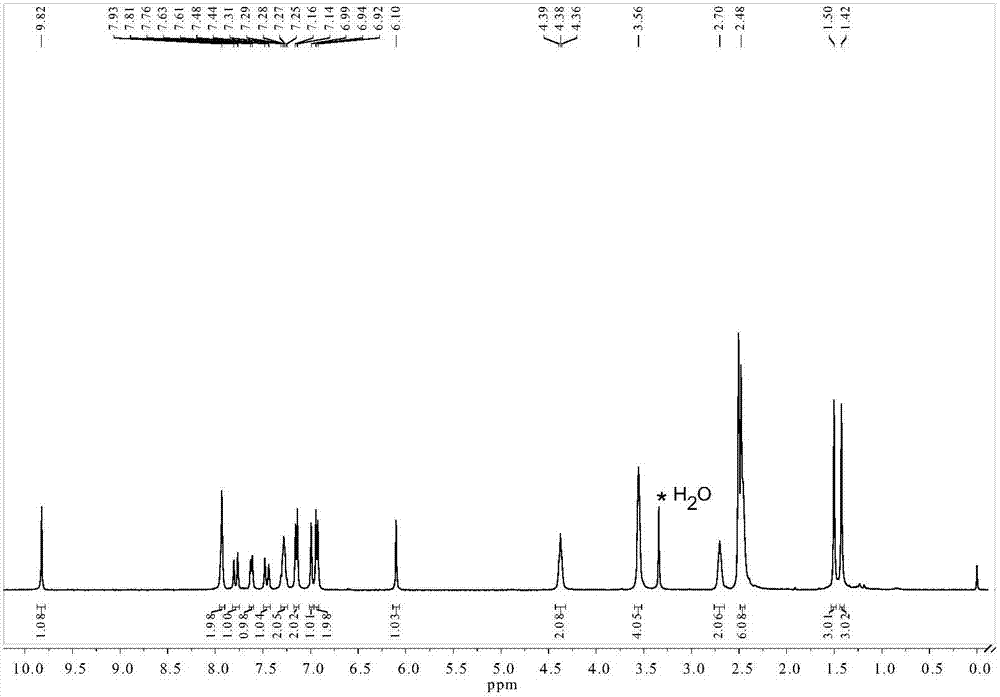
[0035] 1 H NMR (400MHz, DMSO-d 6 ): δ9.82(s,1H),7.93(s,2H),7.79(d,J=16.4Hz,1H),7.62(d,J=7.2Hz,1H),7.46(d,J=16.4Hz ,1H),7.28(m,2H),7.15(d,J=8.2Hz,2H),6.99(s,1H),6.93(d,J=8.2Hz,2H),6.10(s,1H),4.38 (t, J=5.7Hz, 2H), 3.56(s, 4H), 2.70(s, 2H), 2.48(s, 6H), 1.50(s, 3H), 1.42(s, 3H).
[0036] 13 C NMR (100MHz, DMSO-d 6 ):δ158.47,155.61,151.25,143.37,139.94, 139.55,137.75,133.39,132.80,131.22,129.87,125.95,125.19,123.12,...
Embodiment 2
[0040] Weigh compound I and morpholine indole aldehyde in a three-necked flask, the molar ratio is 1:5.5, and add 30ml of anhydrous ethylene glycol methyl ether as a solvent. Then, nitrogen was introduced, and piperidine and glacial acetic acid were added in sequence after the reaction was heated to 140°C. After the reaction mixture was refluxed for 24 hours under a nitrogen atmosphere, the solvent was distilled off under reduced pressure, and the resulting residue was subjected to column chromatography (CH 2 Cl 2 / C 2 h 5 OH=40 / 1) to obtain the target dye 2 as a dark green solid.
[0041] 1 H NMR (400MHz, DMSO-d 6 ):δ9.81(s,1H),8.07(d,J=7.6Hz,2H),7.88(s,2H),7.65(m,6H),7.32(dt,7.6Hz,4H),7.19(d ,J=8.4Hz,2H),6.94(m,4H),4.38(t,J=6.4Hz,4H),3.56(t,J=4.8Hz,8H),2.72(t,J=6.4Hz,4H ),2.47(s,8H),1.51(s,6H).
[0042] 13 C NMR (125MHz, DMSO-d 6 ):δ158.44,152.83,140.98,137.93,136.85, 133.03,132.97,130.66,130.27,125.93,125.53,123.08,121.16,120.42,117.29, 116.34,114.63,114.11,111.43...
Embodiment 3
[0046] Weigh compound II and morpholine indole aldehyde into a three-necked flask, the molar ratio is 1:3, and add 30ml of anhydrous toluene as a solvent. Then, nitrogen was introduced, and piperidine and glacial acetic acid were added in sequence after the reaction was heated to 110°C. After the reaction mixture was refluxed under nitrogen atmosphere for 4 hours, the solvent was distilled off under reduced pressure, and the obtained residue was subjected to column chromatography (CH 2 Cl 2 / C 2 h 5 OH=55 / 1) to obtain the target dye 3 as a dark blue solid.
[0047] 1 H NMR (400MHz, DMSO-d 6 ):δ8.10(s,1H),7.91(dd,1H),7.22(d,1H),7.14(d,1H),7.05(s,1H),6.99(s,2H),6.87(dd, 1H),6.10(s,1H),5.70(s,1H),3.95(t,1H),3,64(t,2H),2.66(t,1H),2.48(t,2H),2.16(s ,1H),2.08(s,2H).
[0048] 13 C NMR (100MHz, DMSO-d 6 ):δ159.28,153.16,150.19,146.92,145.12, 136.68,133.42,132.08,129.89,128.28,125.94,125.02,122.56,122.22,121.56, 120.78,119.53,114.54,110.96,66.79,57.06,53.10,46.57,16.08, 12.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com