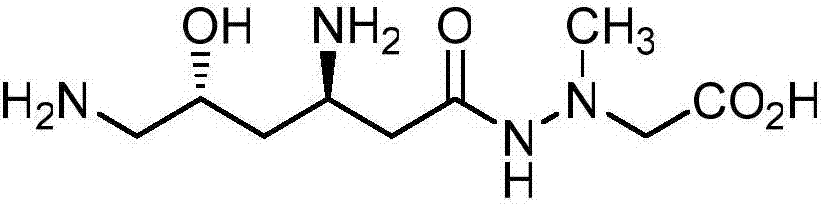
Full-synthesizing method of natural product (+)-negamycin
A natural product and total synthesis technology, applied in the fields of compounds, chemical instruments and methods, and preparation of hydrazides of group 4/14 elements of the periodic table, can solve the problems affecting the structure-activity relationship and limit the research and synthesis of negamycin medicinal chemistry Long route and other problems, to achieve the effect of short production cycle and sufficient source of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
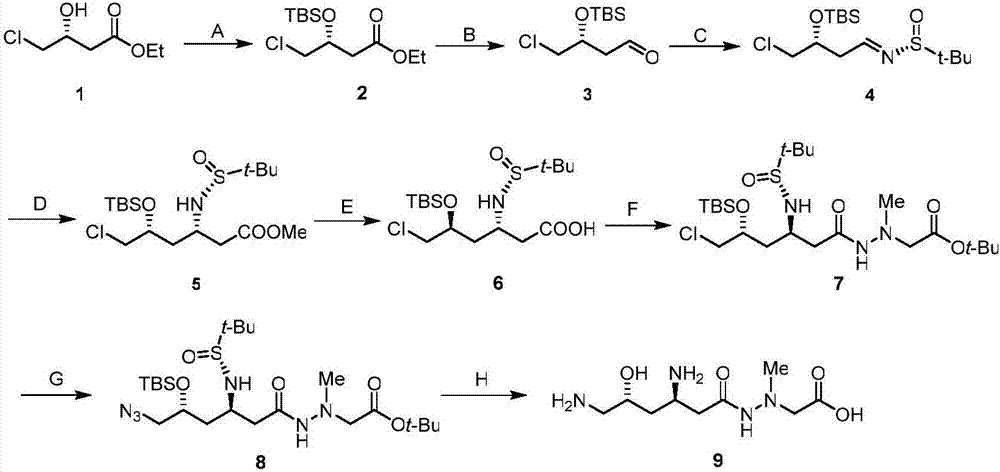
Embodiment 1
[0035] Synthesis of (R)-3-tert-butyldimethylsiloxy-4-chlorobutyric acid ethyl ester (2) in step A
[0036] 2.72g of imidazole was dissolved in 20mL of DMF, cooled to 0°C in an ice-water bath, 3.3g of compound 1 and 3.0g of tert-butyldimethylsilicon chloride were added in sequence, and after stirring for 24h, the reaction was monitored by TLC (developing solvent, Petroleum ether: ethyl acetate=5:1, v / v), add 5 mL of saturated aqueous sodium bicarbonate solution to quench the reaction, add 20 mL of water and stir well, separate the organic phase, extract the aqueous phase with ether (3×30 mL), combine the organic The phase was washed with saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, filtered, concentrated, purified by column chromatography, eluent (petroleum ether:ethyl acetate=100:1) to obtain 5.0 g of yellow oil, yield 90%.
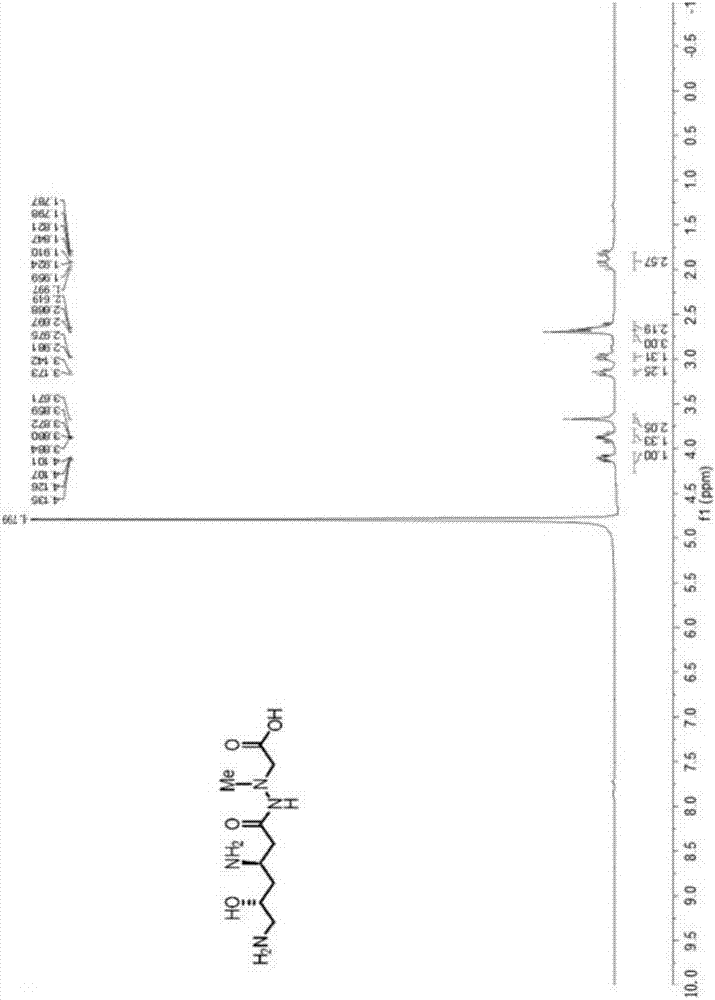
[0037] Specific rotation value [α] D 25 18.0(c 1.03, CHCl 3 ); the H NMR spectral data of the compound are as fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com