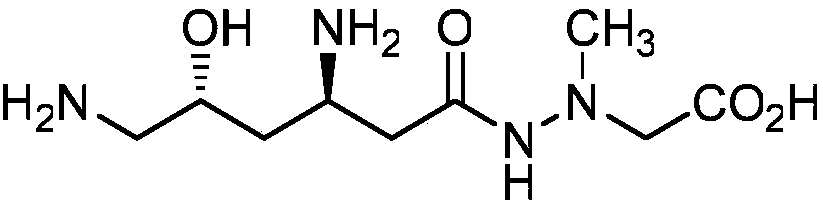
A kind of total synthesis method of natural product (+)-negamycin
A natural product and total synthesis technology, applied in the fields of compounds, chemical instruments and methods, organic chemistry, etc. of group 4/14 elements of the periodic table, can solve the problems affecting the structure-activity relationship, restricting the research of negamycin medicinal chemistry, and synthetic routes Lengthy and other problems, to achieve the effect of short production cycle and sufficient source of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
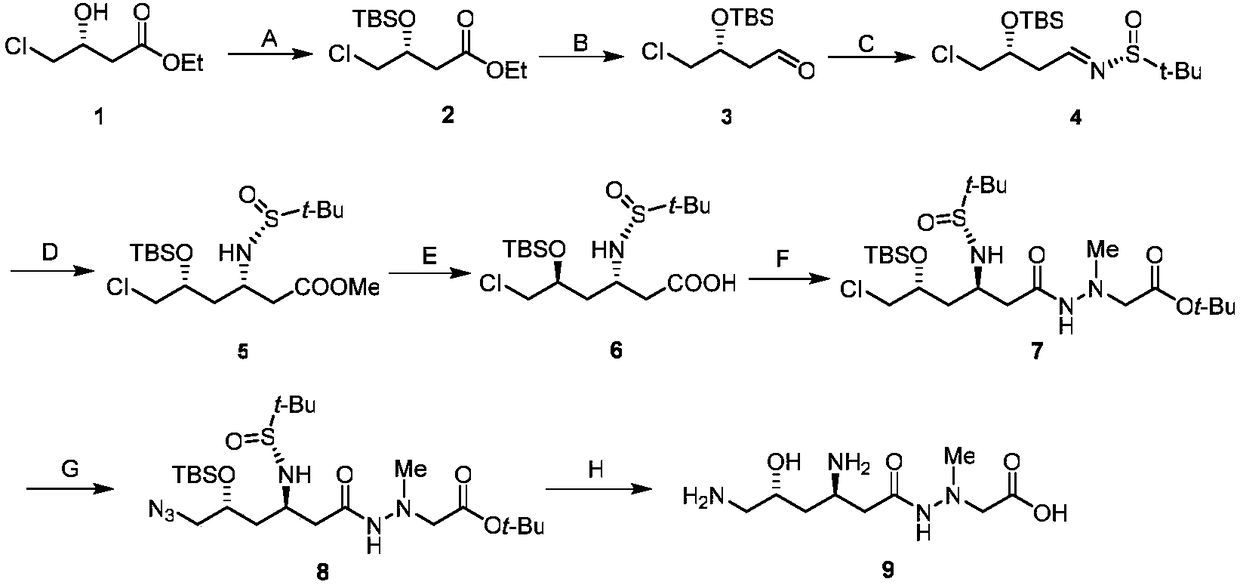
Embodiment 1
[0035] Synthesis of (R)-3-tert-butyldimethylsilyloxy-4-chlorobutyric acid ethyl ester (2) in step A
[0036] Dissolve 2.72g of imidazole in 20mL of DMF, cool to 0°C in an ice-water bath, then add 3.3g of compound 1 and 3.0g of tert-butyldimethylsilyl chloride in sequence, stir and react for 24h, then monitor the reaction by TLC (developing solvent, Petroleum ether: ethyl acetate=5:1, v / v), add 5mL saturated aqueous sodium bicarbonate solution to quench the reaction, add 20mL water and stir well, separate the organic phase, extract the aqueous phase with diethyl ether (3×30mL), combine the organic Phase, washed with saturated aqueous sodium chloride, dried over anhydrous sodium sulfate, filtered, concentrated, purified by column chromatography, eluent (petroleum ether: ethyl acetate = 100:1), to obtain 5.0 g of yellow oil, the yield 90%.
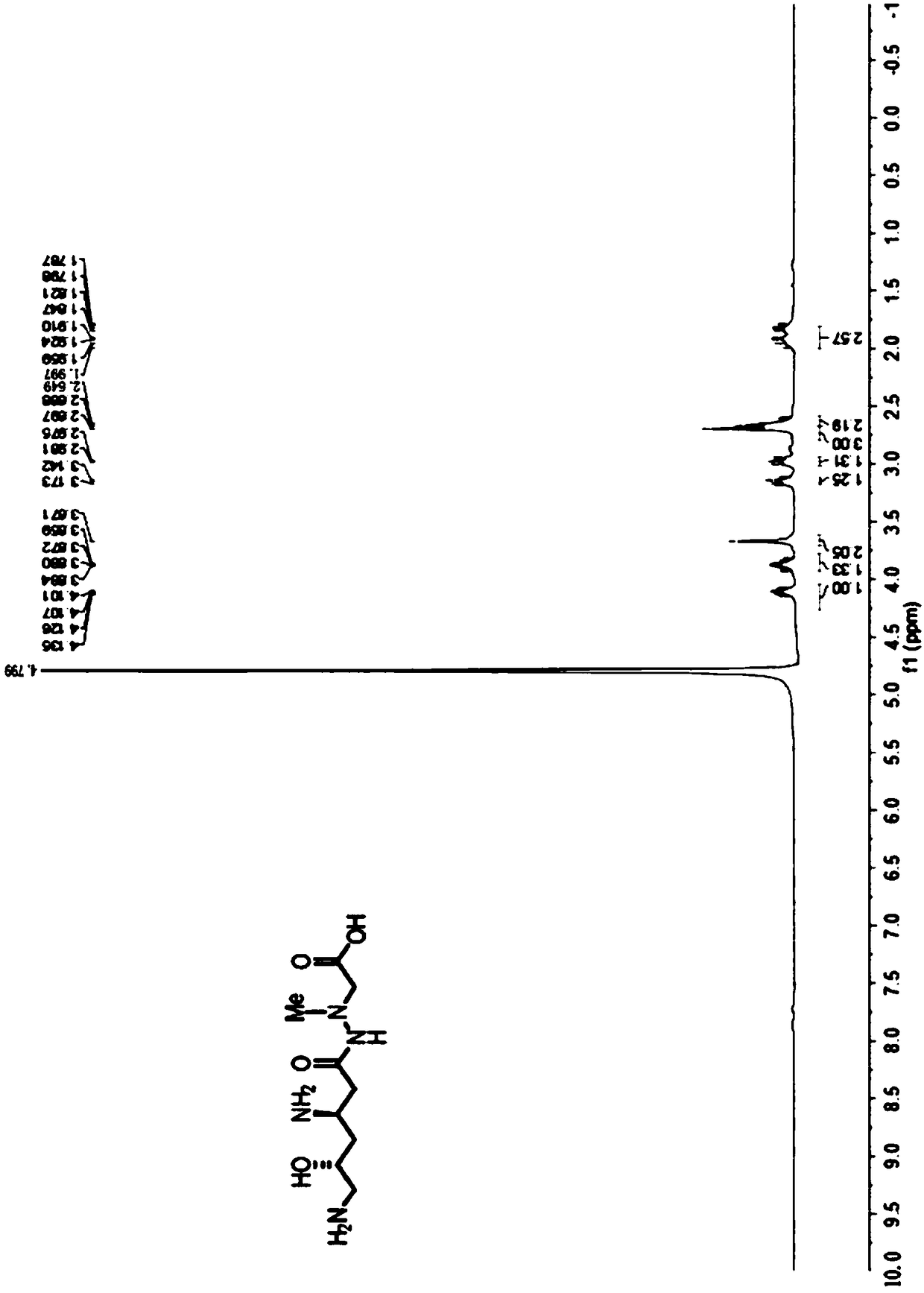
[0037] Specific rotation value [α] D 25 18.0 (c 1.03, CHCl 3 ); The proton nuclear magnetic resonance spectrogram data of compound are ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com