Listeria enriched culture medium and preparation method
A Listeria and culture medium technology, applied in the field of microbiology, can solve the problems of the existence of non-pathogenic Listeria and other environmental bacteria, hidden dangers to human health, cumbersome and time-consuming problems, and achieve shortened culture time, improved sensitivity, and damage The effect of diminishing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1. Comparison of utilization of biochemical substrates by pathogenic Listeria and harmless Listeria
[0030] 1. Strains
[0031] Listeria monocytogenes reference strain (ATCC-BAA-678, EGD-e), Listeria harmless reference strain (ATCC-BAA-680, LIN), Listeria eziri reference strain (ATCC-BAA-679, PAM).
[0032] 2. Reagents
[0033] Reagents required for BIOLOG experiments, including magnesium chloride, calcium chloride, arginine, glutamic acid, L-cystine, 5'-uridine sodium, yeast extract, Tween-80, double distilled water, BIOLOG color development Dye Dye mix-F.
[0034] 3. Culture medium preparation
[0035] Magnesium chloride, calcium chloride, arginine, glutamic acid, L-cystine, sodium 5'-uridine, yeast extract, Tween-80, and double distilled water were prepared as additives according to the operating procedures provided by BIOLOG. Then the additives were mixed with the culture solution IF-0aGN / GP provided by BIOLOG company and the chromogenic dye Dye mix-F ...
Embodiment 2
[0044] Embodiment 2. Design and preparation of basic enrichment medium
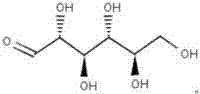
[0045] According to the experimental results of Example 1, β-D-allose was used as the carbohydrate of the medium, and on the basis of adding other nutrients and chemical components, the following basal medium formula was designed and prepared:
[0046]
[0047]
[0048] Dissolve the above components in 1000ml distilled water, adjust the pH value to 7.2, mix well, sterilize at 121°C for 15min, cool and store at 4°C for later use.
Embodiment 3
[0049] Example 3. The growth rate comparison of bacteria in the basic enrichment medium
[0050] 1. Strains
[0051] Listeria monocytogenes reference strain (ATCC-BAA-678, EGD-e), Listeria harmless reference strain (ATCC-BAA-680, LIN), Listeria eziri reference strain (ATCC-BAA-679, PAM).
[0052] 2. Reagents
[0053] With embodiment 2.
[0054] 3. Culture medium preparation
[0055] Prepared according to Example 2.
[0056] 4. Bacterial Culture
[0057] Listeria monocytogenes reference strain (ATCC-BAA-678, EGD-e), Listeria harmless reference strain (ATCC-BAA-680, LIN), Listeria eziri reference strain (ATCC-BAA-679, PAM) was cultured in liquid brain-heart medium to OD=0.6, and then diluted to a specific concentration with phosphate buffered saline (PBS).
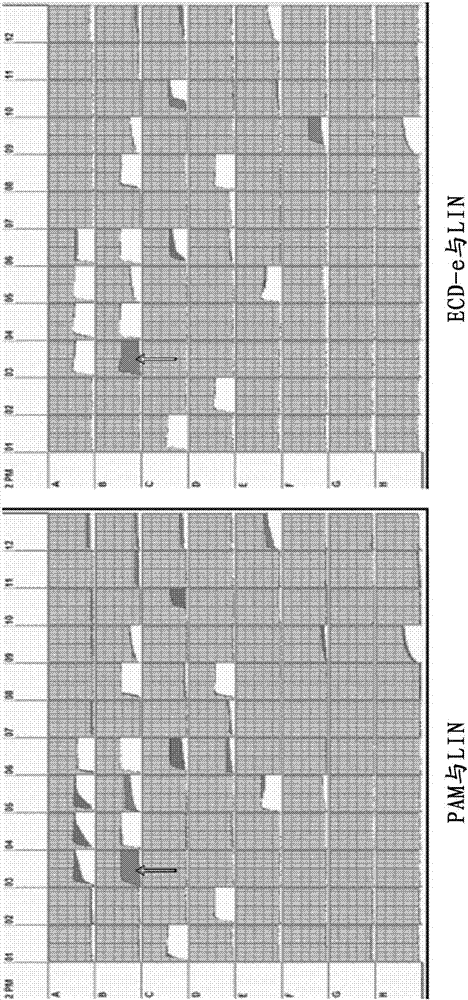
[0058] 5. Growth Rate Determination
[0059] Inoculate the same amount of Listeria monocytogenes, Listeria eziri, and Listeria innocua into three OD tubes containing 5ml of allose-based enrichment medium, and take ou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com