Antigen-specific T lymphocyte freezing medium and preparation method and application thereof
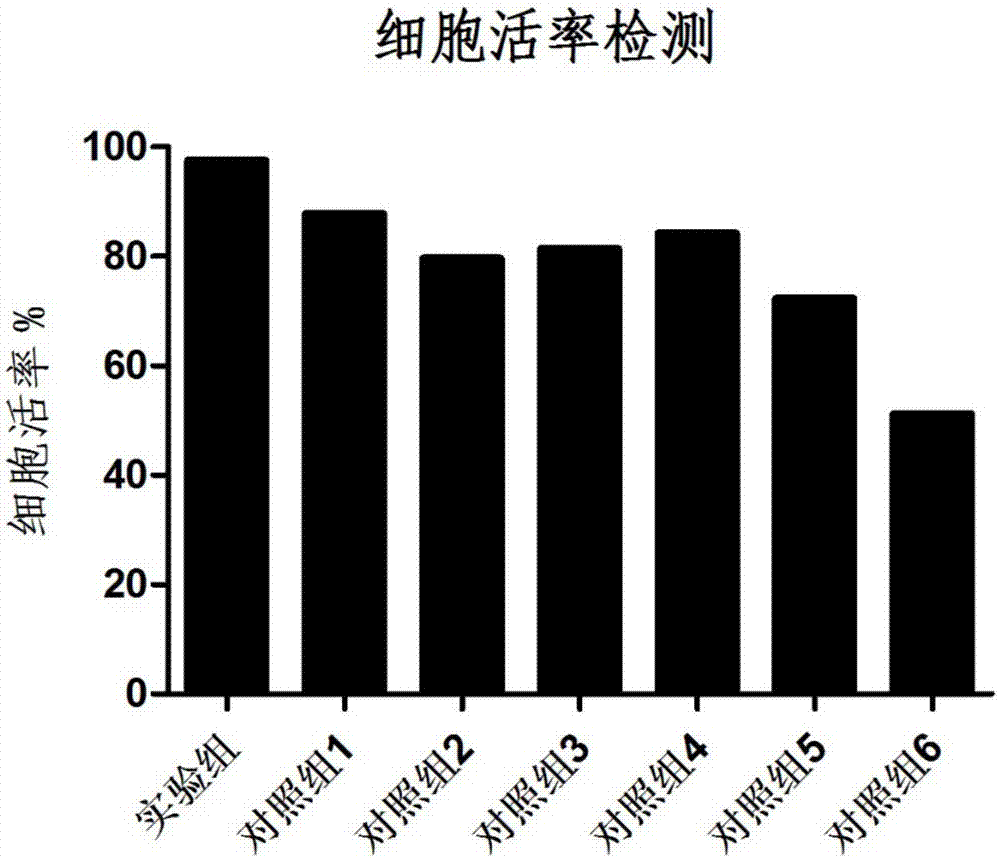
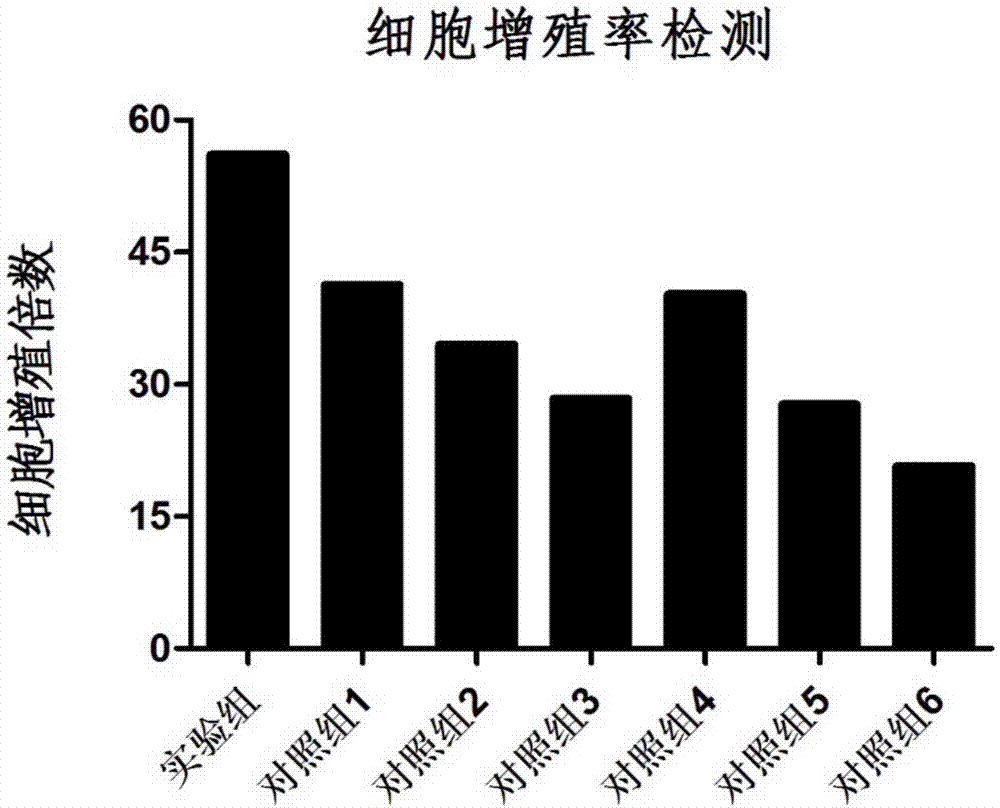
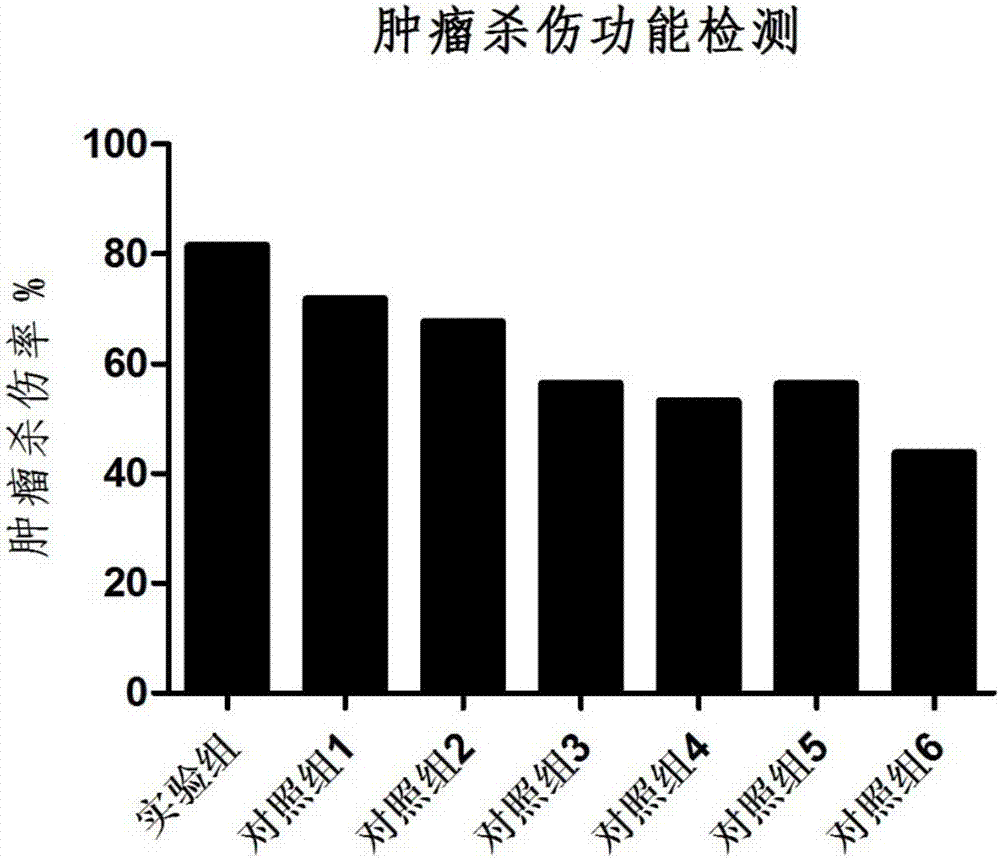
A lymphocyte and cryopreservation technology, applied in the field of antigen-specific T lymphocyte cryopreservation and its preparation, can solve the problems of restricting the clinical application of cryopreserved cells, low cell killing function, insufficient cell proliferation, etc. Tumor killing function, the effect of maintaining the survival rate of resuscitation, and improving the cell viability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] The second aspect of the present invention provides a method for preparing an antigen-specific T lymphocyte cryopreservation solution, comprising:
[0067] Bomaili Electrolyte Injection, Glucose Sodium Chloride Injection, Dextran Glucose Injection and Human Serum Albumin Solution are 30%-40%, 30%-40%, 5%-15% and 15%- Mix at a ratio of 25% to prepare freezing solution A;
[0068] Bomaili Electrolyte Injection, Glucose Sodium Chloride Injection, Dextran Glucose Injection, Human Serum Albumin Solution and Dimethyl Sulfoxide are 20%-30%, 20%-30%, 5%- 15%, 15%-25% and 10%-20% were mixed to prepare freezing solution B;
[0069] Freezing solution A and freezing solution B are stored separately. When used, the freezing solution A and freezing solution B are mixed according to the volume ratio of 1:0.5-2 to obtain the freezing solution.
[0070] In the present invention, cryopreservation solution A and cryopreservation solution B are mixed according to a volume ratio of 1:1. ...
Embodiment 1
[0094] A cryopreservation solution for antigen-specific T lymphocytes, including cryopreservation solution A and cryopreservation solution B, where the cryopreservation solution A includes the following volume fraction components:
[0095] 35% Bomaili Electrolyte Injection, 35% Glucose Sodium Chloride Injection, 10% Dextran Glucose Injection and 20% Human Serum Albumin Solution;
[0096] Freezing solution B includes the following components by volume fraction:
[0097] 27.5% Bomaili Electrolyte Injection, 27.5% Glucose Sodium Chloride Injection, 10% Dextran Glucose Injection, 20% Human Serum Albumin Solution and 15% Dimethyl Sulfoxide;
[0098] Below are manufacturer's brand and article number of each raw material that adopts in the embodiment of the present invention:
[0099] Table 1 Raw material list
[0100]
[0101] The glucose sodium chloride injection contains 5% dextrose and 0.45% sodium chloride, and the dextran glucose injection contains 10% low molecular weight...
Embodiment 2
[0104] A cryopreservation solution for antigen-specific T lymphocytes, including cryopreservation solution A and cryopreservation solution B, where the cryopreservation solution A includes the following volume fraction components:
[0105] 30% Bomaili Electrolyte Injection, 40% Glucose Sodium Chloride Injection, 5% Dextran Glucose Injection and 25% Human Serum Albumin Solution;
[0106] Freezing solution B includes the following components by volume fraction:
[0107] 30% Bomaili Electrolyte Injection, 20% Glucose Sodium Chloride Injection, 5% Dextran Glucose Injection, 25% Human Serum Albumin Solution and 20% Dimethyl Sulfoxide;
[0108] The glucose sodium chloride injection contains 5% dextrose and 0.45% sodium chloride, and the dextran glucose injection contains 10% low molecular weight dextran and 5% dextrose;
[0109] Cryopreservation solution A and cryopreservation solution B are stored separately. When in use, cryopreservation solution A and cryopreservation solution B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com