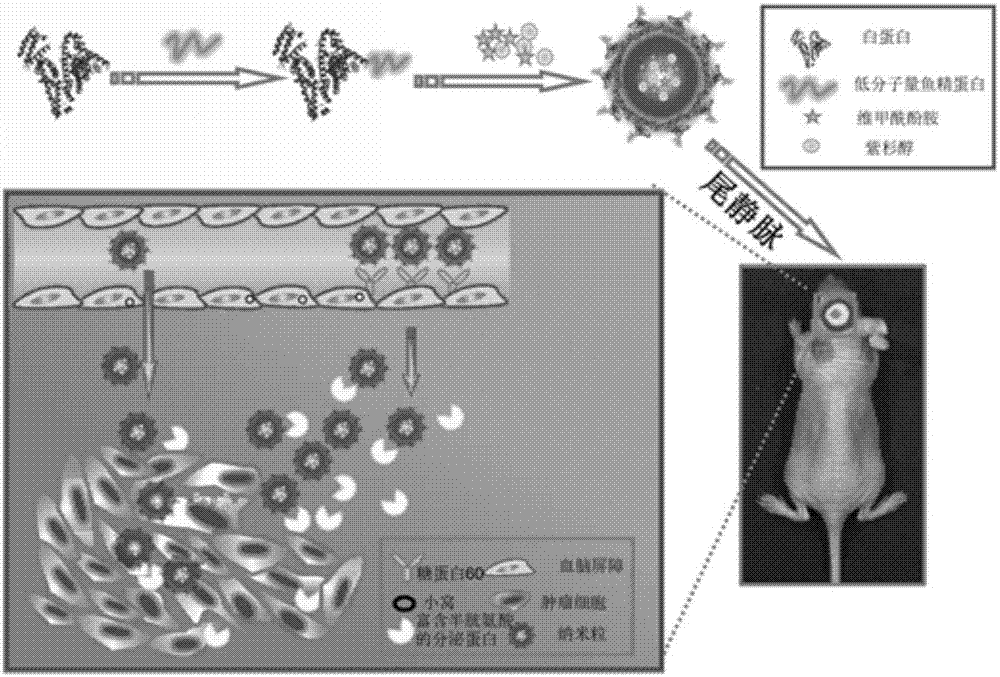
Albumin nano-particles and preparation method and use thereof
An albumin nanoparticle and albumin technology, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of poor water solubility of paclitaxel, allergic reactions in patients with castor oil, etc. The effect of enhancing drug-carrying capacity and promoting cell apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0059] Preparation Example 1: Synthesis of Low Molecular Weight Protamine Modified Albumin
[0060] 1. Activation of Albumin
[0061] Weigh 5 mg SMCC and dissolve it in 200 μl of anhydrous DMSO, weigh 200 mg bovine serum albumin (BSA) and dissolve it in the phosphate buffered saline solution of pH 7.2, after the dissolution is complete, add the SMCC solution dropwise to the BSA solution, After stirring at room temperature for 1 h, the product was purified by FPLC molecular sieves with a detection wavelength of 280 nm and a flow rate of 1 ml / min. First connect Desalting and FPLC, use 0.1M phosphate buffer (pH7.2) as the mobile phase to equilibrate the column for about 4-5 column volumes, that is, 40-50ml, then inject the reaction product into the injection loop, and After the ultraviolet baseline is adjusted to zero, the sample enters the Desalting at a speed of 1ml / min, and after about 3 minutes, the eluted ultraviolet peak is collected by the instrument through the program s...
preparation Embodiment 2
[0066] Preparation Example 2: Preparation of Albumin Nanoparticles and Albumin Nanoparticles Modified by Low Molecular Weight Protamine
[0067] Prepare the urea solution:
[0068] Dissolve 3.0g Tris base and 28.0g urea in water, adjust the pH to 8.3 with concentrated hydrochloric acid, and set aside. Weigh 100mg of BSA and the above-prepared BSA-LMWP, dissolve them in 400μl of water, transfer them to 5.6ml of urea solution and mix them evenly, then add 1.05ml of absolute ethanol and mix them evenly, add sodium borohydride solution, and finally The concentration range is 0.01-0.1mol / L, and then centrifuged at 8000rpm for 3min, followed by 30min at room temperature, 30min in a 50°C water bath, and placed at room temperature for later use to prepare BSA-denatured urea solution and BSA-LMWP-denatured urea solution.
[0069] Preparation of blank nanoparticles:
[0070] Take 0.1ml of absolute ethanol and add it dropwise to the above BSA-denatured urea solution or BSA-LMWP denatur...
experiment Embodiment 1
[0073] Experimental Example 1: In vitro characterization experiments of albumin nanoparticles and albumin nanoparticles modified with low molecular weight protamine
[0074] (1) Particle size and potential
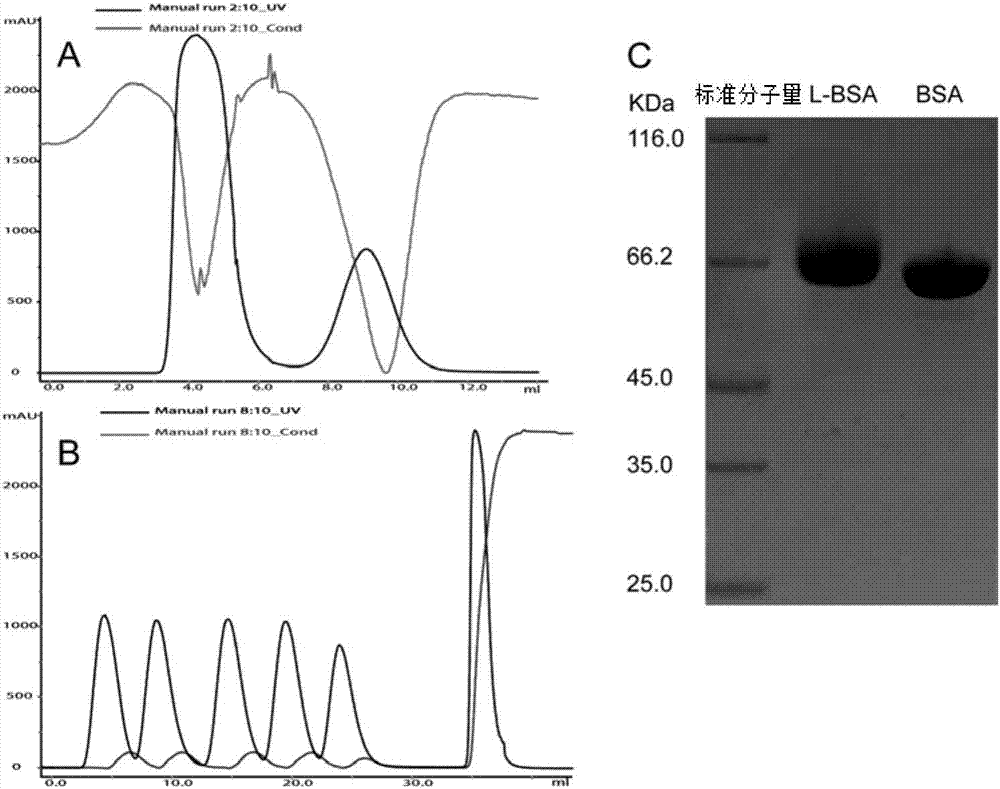
[0075] Take 100 μl each of purified albumin nanoparticles (BSA NP) and low molecular weight protamine-modified albumin nanoparticles (L-BSA NP), dilute with purified water to a final volume of 1ml, and use a Malvern particle size analyzer Determination of particle size and potential, the results are as follows image 3 with Figure 5 shown.
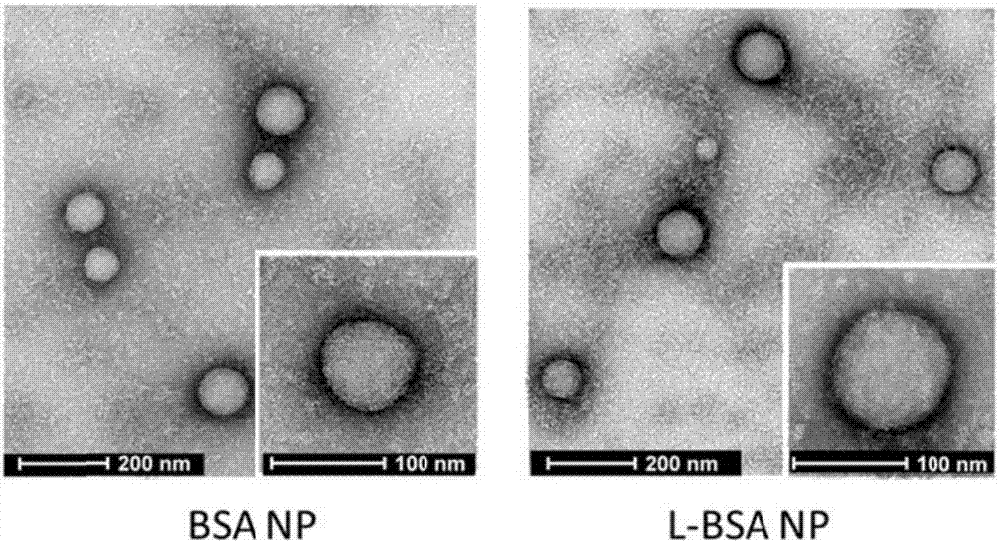
[0076] (2)TEM
[0077] Add albumin nanoparticles (BSA NP) and low-molecular-weight protamine-modified albumin nanoparticles (L-BSA NP) onto the pre-hydrated copper grid respectively, blot dry after 1 min, and add acetate glaze for negative staining Let it dry naturally after 1 min. Determination and analysis of albumin nanoparticles by transmission electron microscope, the results are as follows: Figure 4 shown.
[0078] (3) I...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com