Silk fibroin nanoparticles and drug-loading silk fibroin nanoparticles
A technology of silk fibroin and nanoparticles, which is applied in the field of medicine, can solve the problems that drugs are difficult to reach the retinal therapeutic effect, low eye bioavailability, and large systemic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
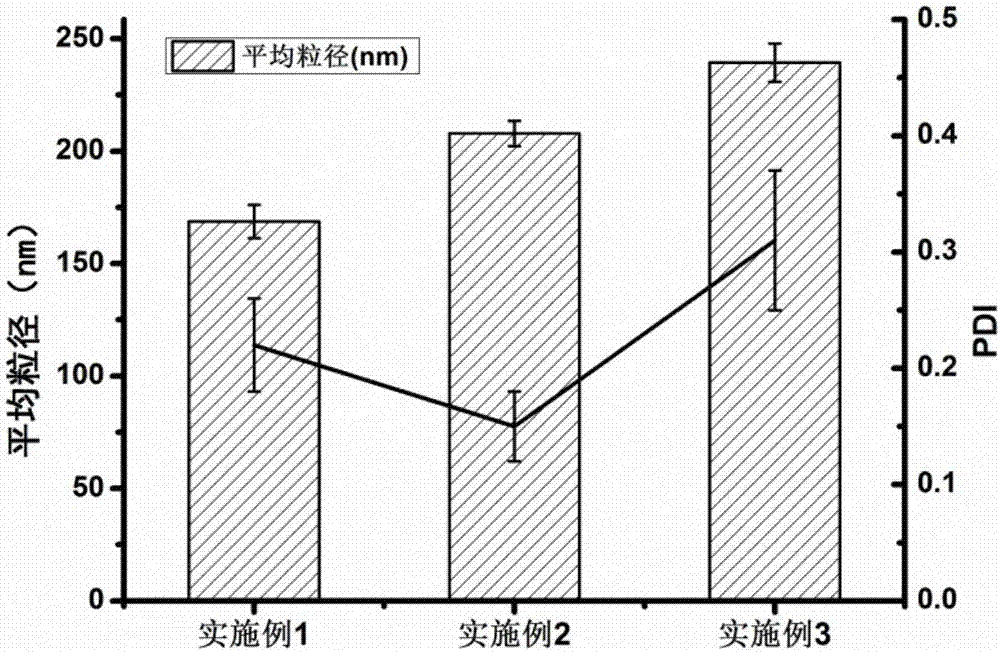
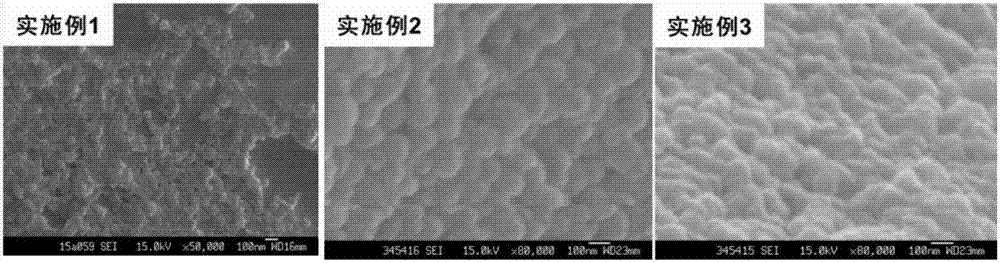
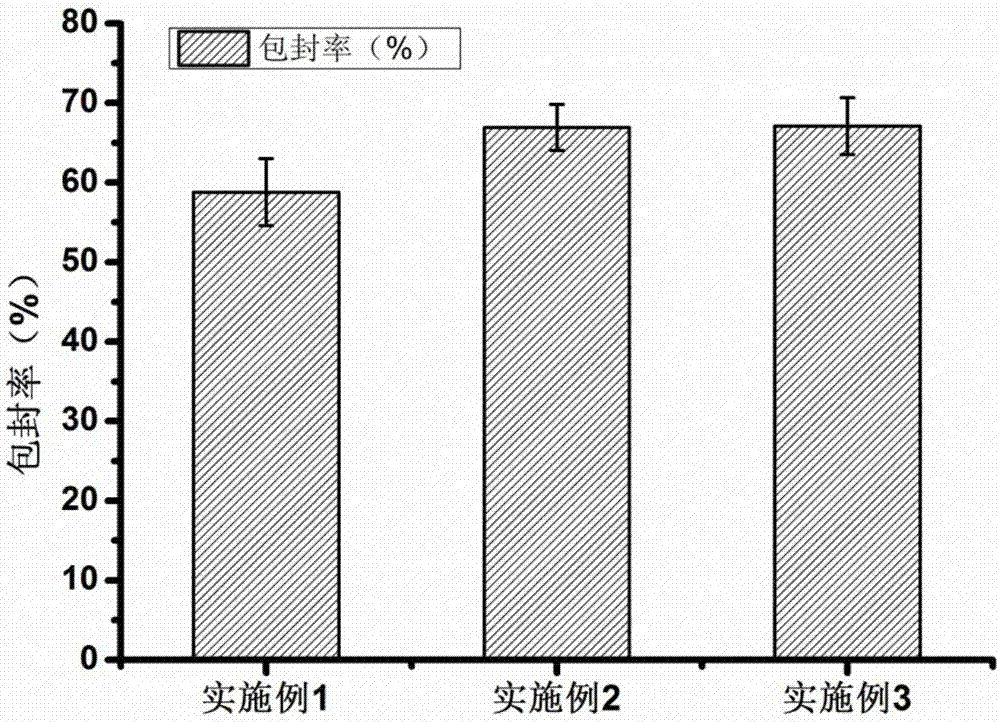
Embodiment 1
[0067] 1. Preparation of silk fibroin nanoparticles
[0068] The silk fibroin material was dissolved in deionized water, stirred continuously until the silk fibroin was completely dissolved, and prepared into a silk fibroin solution with a concentration of 1.0% (w / v), and stood at 4° C. for 2 hours. Use a syringe to extract the above silk fibroin solution, and quickly inject it into a constant temperature ethanol solution at 60°C. The volume ratio (v / v) of ethanol to silk fibroin solution is 4:1. At the same time, use a magnetic stirrer to continuously stir the mixture until it forms Milky white nanoparticle suspension, continue to stir at constant temperature for 30 minutes. After the nanoparticles are completely formed, transfer the above nanosuspension to a centrifuge tube, centrifuge at 16,000 rpm for 30 min at 4°C, discard the supernatant, redisperse and wash the precipitate with deionized water, and centrifuge again to separate the nanoparticles, repeat This operation i...
Embodiment 2
[0077] 1. Preparation of silk fibroin nanoparticles
[0078] The silk fibroin material was dissolved in deionized water, stirred continuously until the silk fibroin was completely dissolved, and prepared into a silk fibroin solution with a concentration of 2.5% (w / v), and stood at 4° C. for 4 hours. Use a syringe to extract the above silk fibroin solution, and quickly inject it into acetone solution at a constant temperature of 40°C. The volume ratio (v / v) of acetone to silk fibroin solution is 6:1. Milky white nanoparticle suspension, continue to stir at constant temperature for 30 minutes. After the nanoparticles are completely formed, transfer the above nanosuspension to a centrifuge tube, centrifuge at 20,000 rpm for 30 min at 4°C, discard the supernatant, redisperse and wash the precipitate with deionized water, and centrifuge again to separate the nanoparticles, repeat This operation is performed 3 times. Finally, add quantitative deionized water to the above-mentioned...
Embodiment 3
[0087] 1. Preparation of silk fibroin nanoparticles
[0088] The silk fibroin material was dissolved in deionized water, stirred continuously until the silk fibroin was completely dissolved, and prepared into a silk fibroin solution with a concentration of 5.0% (w / v), and stood at 4° C. for 8 hours. Use a syringe to extract the above silk fibroin solution, and quickly inject it into isopropanol solution at a constant temperature of 20°C. The volume ratio (v / v) of isopropanol to silk fibroin solution is 8:1. At the same time, use a magnetic stirrer to continuously stir and mix solution until a milky white nanoparticle suspension is formed, and continue stirring at constant temperature for 30 min. After the nanoparticles are completely formed, transfer the above nanosuspension to a centrifuge tube, centrifuge at 4°C and 8000rpm for 30min, discard the supernatant, redisperse and wash the precipitate with deionized water, centrifuge again to separate the nanoparticles, repeat Thi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com