Method for synthesizing trifluoromethyl-containing carbamate by using carbon dioxide
A carbamate and trifluoromethyl technology, which is applied in the fields of chemical industry, medicine, and organic synthesis, can solve the problems of carbamate compounds being less reported, and achieve high yield of target products, safe and simple operation, and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
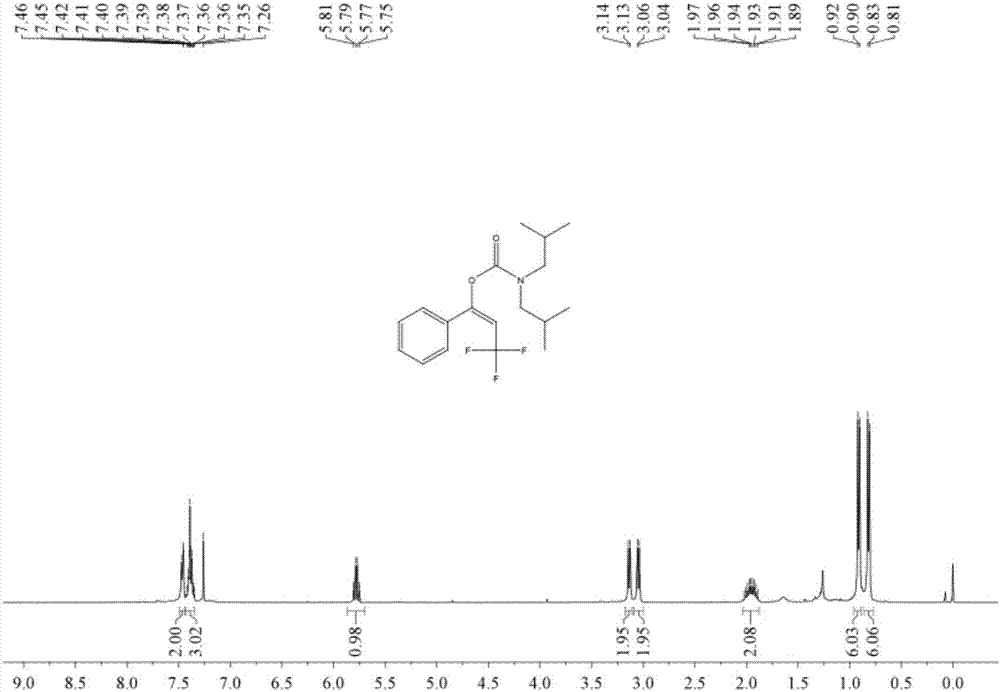
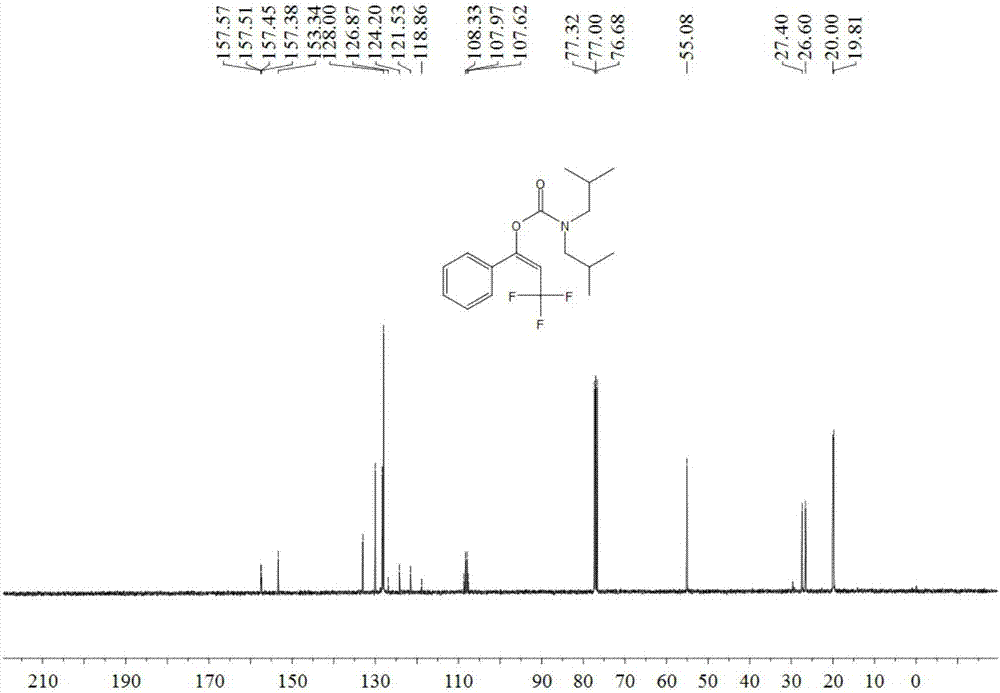
[0050] Add 1.5 ml dimethyl sulfoxide, 0.20 mmol phenylacetylene, 1.0 mmol diisobutylamine, 0.24 mmol 1-trifluoromethyl-1,2-phenyliodyl-3(1H )-ketone, then add 0.02 mmol copper acetate and 0.04 mmol 1,10-phenanthroline, then fill with 4MPa carbon dioxide, stir and react at 60°C for 8 hours, stop heating and stirring, cool to room temperature, and release slowly Release carbon dioxide to atmospheric pressure. The reaction solution was washed with 10 mL of water, extracted three times with ethyl acetate (15 mL each time), the organic phases were combined and dried over anhydrous magnesium sulfate, concentrated by distillation under reduced pressure to obtain a crude product, and then separated and purified by column chromatography to obtain the target product. The column chromatography eluent used was petroleum ether:ethyl acetate mixed solvent with a volume ratio of 2:1, and the yield was 76%.
Embodiment 2
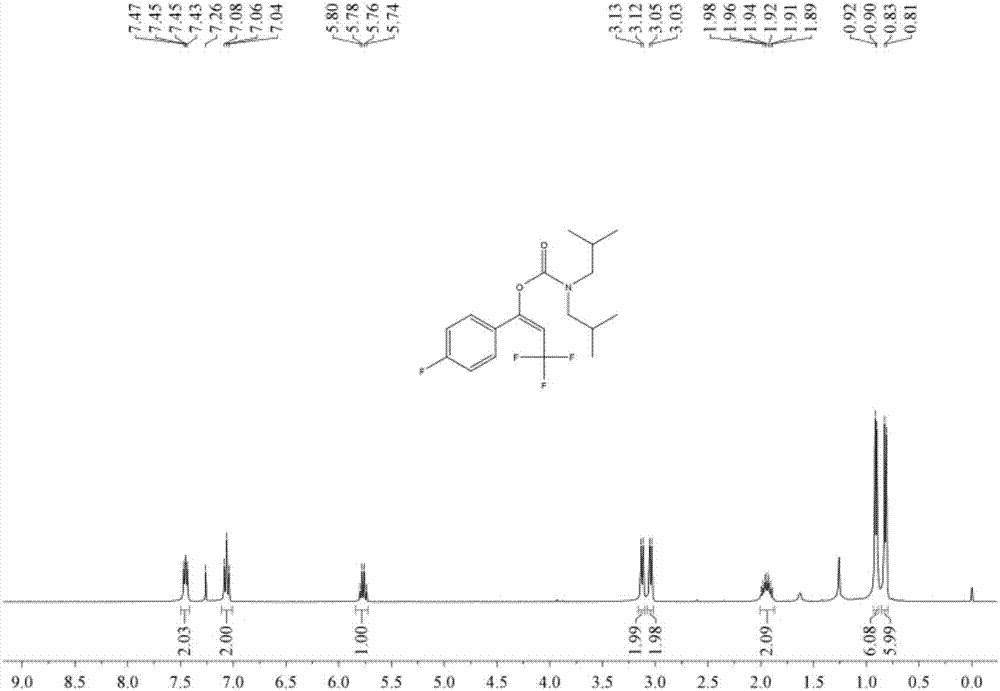
[0052] Add 1.5 ml dimethyl sulfoxide, 0.20 mmol phenylacetylene, 1.0 mmol diisobutylamine, 0.24 mmol 1-trifluoromethyl-1,2-phenyliodyl-3(1H )-ketone, then add 0.02 mmol copper trifluoromethanesulfonate, 0.04 mmol 1,10-phenanthroline, then fill with 4MPa carbon dioxide, stir and react at 60°C for 8 hours, stop heating and stirring, and cool to At room temperature, slowly release carbon dioxide to normal pressure. The reaction solution was washed with 10 mL of water, extracted three times with ethyl acetate (15 mL each time), the organic phases were combined and dried over anhydrous magnesium sulfate, concentrated by distillation under reduced pressure to obtain a crude product, and then separated and purified by column chromatography to obtain the target product. The column chromatography eluent used was petroleum ether:ethyl acetate mixed solvent with a volume ratio of 2:1, and the yield was 71%.
Embodiment 3
[0054] Add 1.5 ml dimethyl sulfoxide, 0.20 mmol phenylacetylene, 1.0 mmol diisobutylamine, 0.24 mmol 1-trifluoromethyl-1,2-phenyliodyl-3(1H )-ketone, then add 0.02 mmol copper acetate, 0.04 mmol 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline, then fill with 4 MPa of carbon dioxide, stir at 60 ° C After reacting for 8 hours, stop heating and stirring, cool to room temperature, and slowly release carbon dioxide to normal pressure. The reaction solution was washed with 10 mL of water, extracted three times with ethyl acetate (15 mL each time), the organic phases were combined and dried over anhydrous magnesium sulfate, concentrated by distillation under reduced pressure to obtain a crude product, and then separated and purified by column chromatography to obtain the target product. The column chromatography eluent used was a mixed solvent of petroleum ether:ethyl acetate with a volume ratio of 2:1, and the yield was 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com