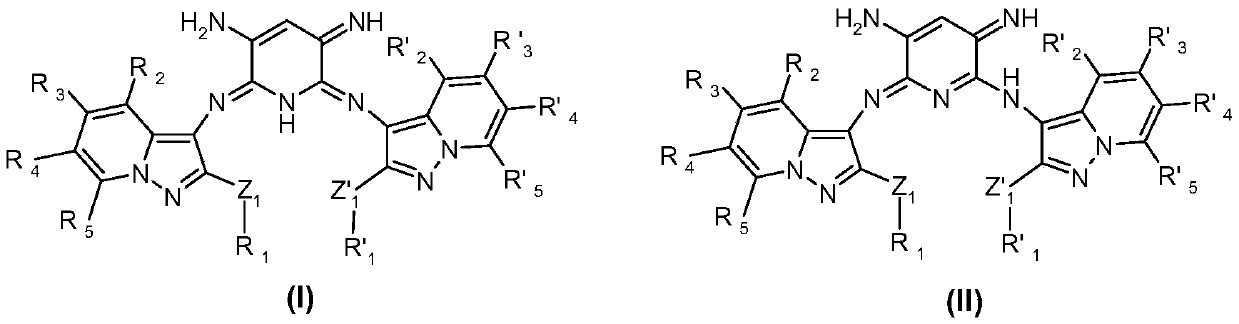
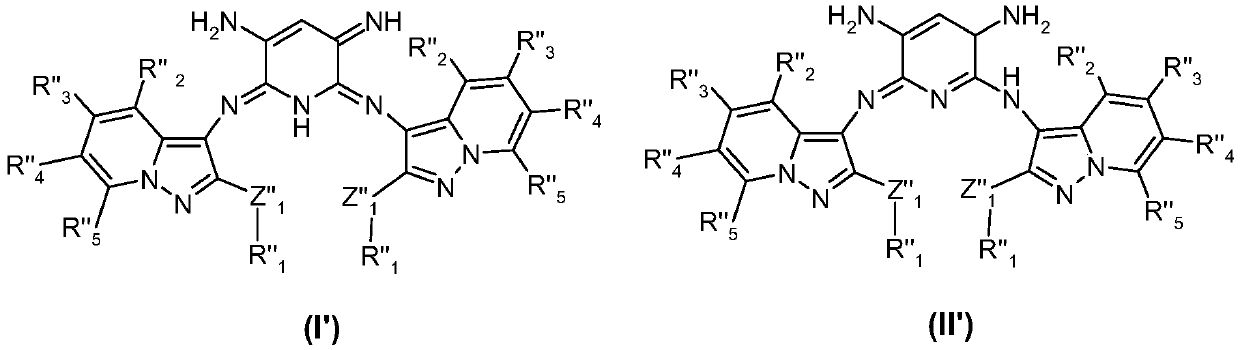
Use of azomethine compounds carrying two pyrazolopyridine units for dyeing keratin fibers
A pyrazolopyridine, azomethine technology, applied in disazomethine dyes, cosmetics, organic chemistry and other directions, can solve the problems of poor relative durability, low resistance to photochemical erosion, weak dyeing power and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
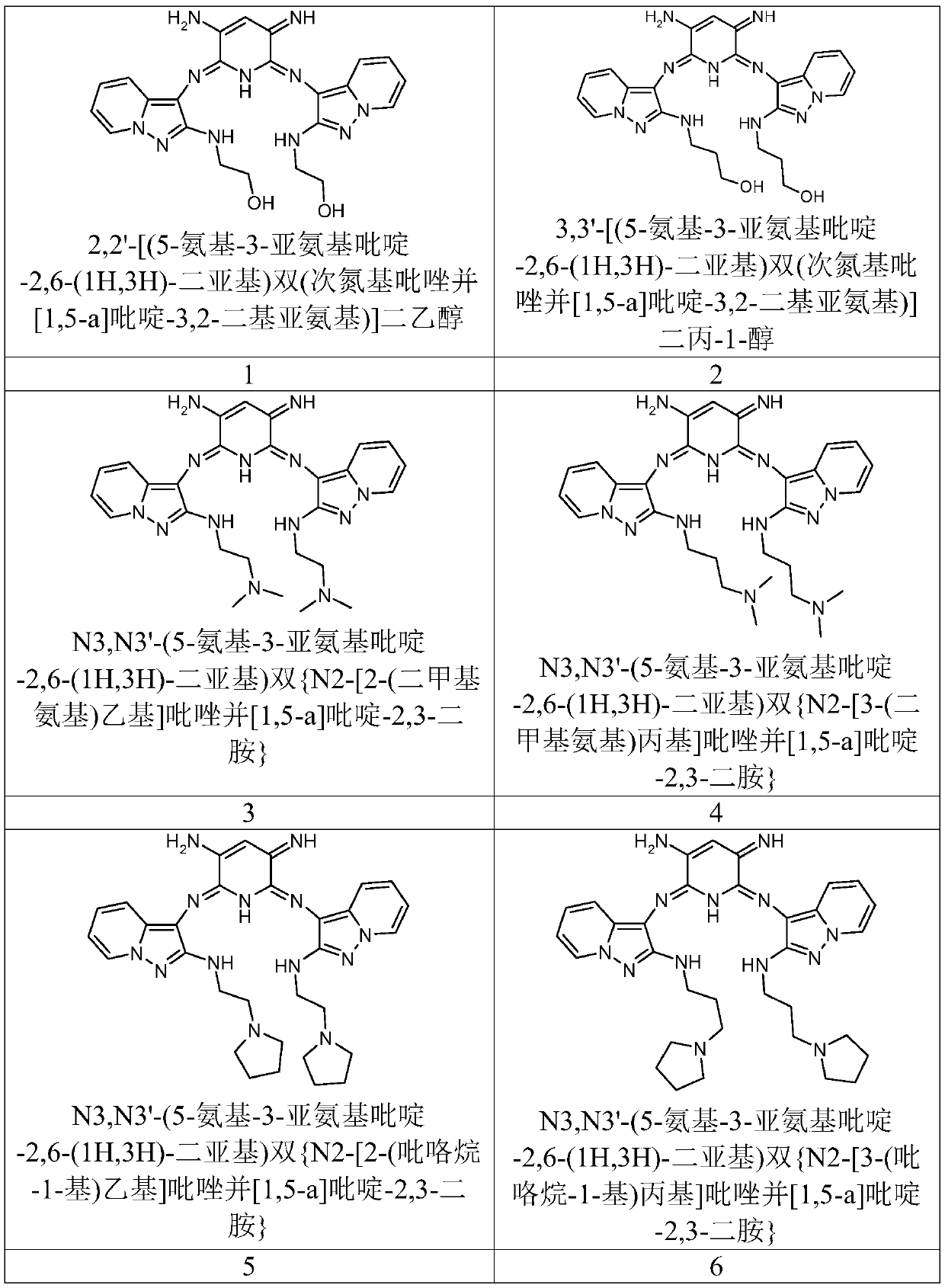
[0199] Example 1 : Synthesis of a dye with the formula:
[0200]
[0201]
[0202] Put 500ml of ethanol into a 1-liter single-neck round bottom flask equipped with a calcium chloride protection tube, then add 50g (0.2177mol) of 2-[(3-aminopyrazolo[1,5-a ]pyridin-2-yl)oxy]ethanol hydrochloride.
[0203] To this solution was added 14.2 g (0.986 mol) of 2-chloropyridine-3,5-diamine and 60.3 ml (0.346 mol) of N-ethyl-N-(propan-2-yl)propan-2-amine .
[0204] The solution thus obtained was stirred at room temperature for 4 days. The black precipitate formed was isolated by filtration, washed with water, and dried in a desiccator under vacuum at 30° C. in the presence of a desiccant to constant weight. A black solid is thus obtained.
[0205] Spectral analysis indicated that the obtained compound corresponded to the above structure.
Embodiment 2
[0206] Example 2 : 3,3-[(5-amino-3-iminopyridine-2,6-(1H,3H)-diylidene)bis(nitrilopyrazolo[1,5-a]pyridine-3, 2-diyloxy group)] the synthesis of dipropan-1-ol hydrochloride:
[0207]
[0208] Put 150ml of ethanol into a 500ml single-neck round bottom flask equipped with a calcium chloride protection tube, then add 10.4g (0.04268mol) of 3-[(3-aminopyrazolo[1,5-a ]pyridin-2-yl)oxy]propan-1-ol hydrochloride.
[0209] To this solution was added 2.78 g (0.01940 mol) of 2-chloropyridine-3,5-diamine and 11.8 ml (0.06790 mol) of N-ethyl-N-(propan-2-yl)propan-2-amine .
[0210] The solution thus obtained was then stirred at room temperature for 4 days. The black precipitate formed was isolated by filtration, washed with water, and dried in a desiccator under vacuum at 30° C. in the presence of a desiccant to constant weight. A solid is thus obtained in the form of a black powder.
[0211] Spectral analysis showed that the obtained compound corresponds to 3,3'-[(5-amino-3-imino...
Embodiment 3
[0212] Example 3 : N,N'-(5-amino-3-iminopyridine-2,6-(1H,3H)-diylidene)bis{2-[2-(dimethylamino)ethoxy]pyrazole Synthesis of [1,5-a]pyridin-3-amine} dihydrochloride:
[0213]
[0214] Put 550ml of ethanol into a 1-liter single-necked round-bottomed flask equipped with a calcium chloride protection tube, then add 55g (0.1876mol) of 2-[2-(dimethylamino)ethoxy]pyridine under stirring Azolo[1,5-a]pyridin-3-amine dihydrochloride.
[0215] To this solution were added 12.24 g (0.085 mol) of 2-chloropyridine-3,5-diamine and 52 ml (0.346 mol) of N-ethyl-N-(propan-2-yl)propan-2-amine.
[0216] The solution thus obtained was stirred at room temperature for 4 days. The viscous black oil obtained after removal of the solvent by evaporation under vacuum was chromatographed on a silica column in normal phase with the eluent consisting of 50% dichloromethane and 50% methanol.
[0217] After removal of the solvent by evaporation under vacuum, the viscous black oil crystallized as a blac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com