Method for preparing 1, 2-pentanediol from n-butanol as raw material
A process method and n-butanol technology, applied in the field of preparing 1,2-pentanediol, can solve the problems of flammable and explosive raw materials, corrosion of formaldehyde environment, pollution, etc., and achieve simple post-processing, simple operation and short time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
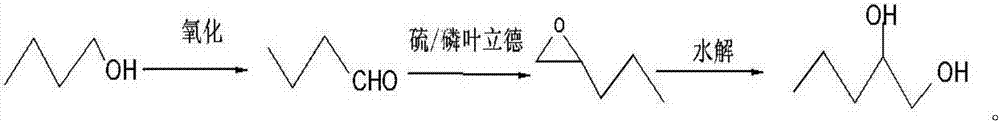
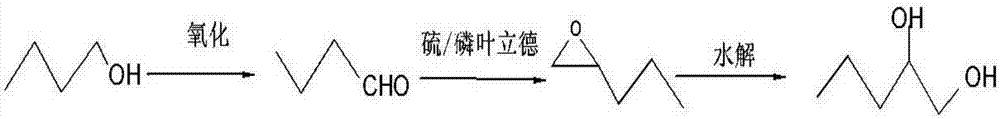
[0019] The synthetic technique of the present invention is as follows:
[0020]
[0021] In the first step, n-butanol is oxidized to prepare n-butyraldehyde
[0022] Its specific synthesis method is: n-butanol is oxidized to prepare n-butyraldehyde: add 0.1mol n-butanol (7.4g), 1mmol TEMPO (0.16g), 34ml dichloromethane, 0.01mol KBr (1.19g) into a 250ml three-necked flask respectively Add it into a three-necked flask after adding 5ml of water, then place it in an ice-water bath at -8°C, and add 0.11mol of sodium hypochlorite aqueous solution (the pH of the sodium hypochlorite solution is 9.5) dropwise with a constant pressure dropping funnel. ℃-30℃, reaction time 5-60min, wash with water, stand to separate the liquid, and remove the solvent under reduced pressure to obtain n-butyraldehyde.
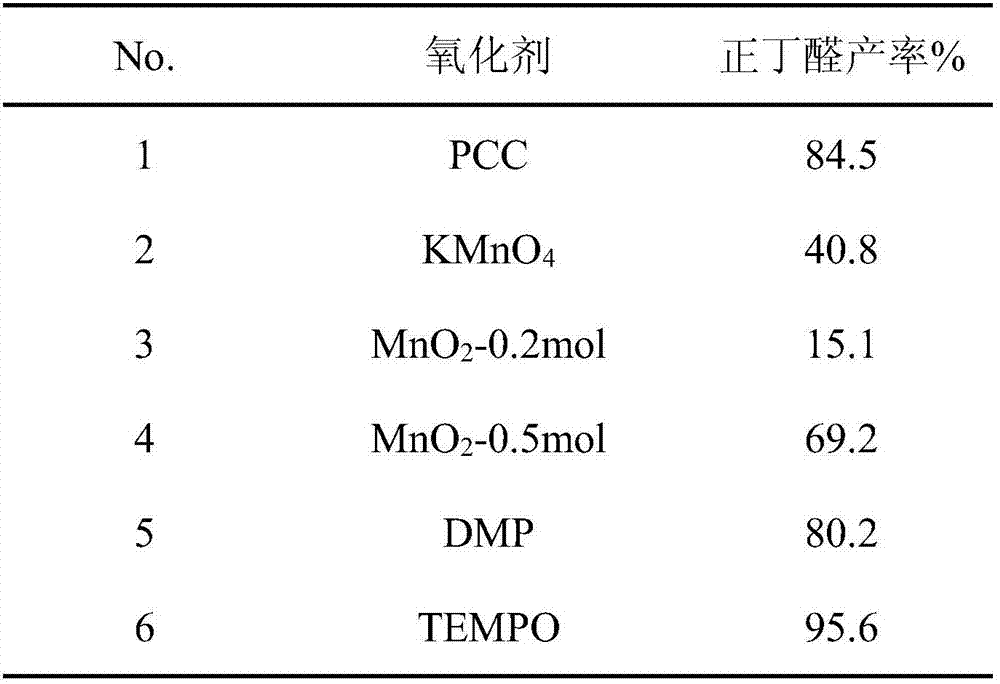
[0023] Using the same conditions as the above-mentioned oxidation method, the difference is that different oxidants are used, and the yield of the product is shown in Table 1.
[0024]...
Embodiment 2
[0027] The second step, the preparation of 1,2-epoxypentane
[0028] Add 0.25 mol of n-butyraldehyde, 0.38 mol of methyl sulfide ylide, potassium carbonate (0.75 mol), and then add 3 mL of dry isopropanol, 0.3 mL of ethanol, and react at room temperature for 12 hours, then add 3 mL of water into a nitrogen-protected reaction flask. Quench the reaction, let stand for liquid separation, dry over anhydrous sodium sulfate, and filter to obtain 1,2-epoxypentane.
[0029] Using the same conditions as the above-mentioned ylide reaction, the difference is that different ylide reagents are used, and the yield of the product is shown in Table 2.
[0030] Table 2
[0031]
Embodiment 3
[0033] The third step, the preparation of 1,2-pentanediol
[0034] Add 5g of potassium hydroxide into 50g of 85% methanol aqueous solution, stir evenly, and heat to reflux, add the separated 1,2-epoxypentane dropwise to the methanol aqueous solution containing a small amount of potassium hydroxide to open the ring, and keep the aqueous solution The pH value is 8, and the 1,2-pentanediol is obtained by static separation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com