Hydrotalcite-like type iron-promoted nickel-based catalyst for hydrogen preparation through autothermal reforming of acetic acid and preparation method
A nickel-based catalyst, autothermal reforming technology, applied in catalyst activation/preparation, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve problems such as catalyst deactivation and achieve stable performance , Inhibit oxidation and ensure stable dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] Weigh 29.969g Ni(NO 3 ) 2 ·6H 2 O, 122.632g Zn(NO 3 ) 2 ·6H 2 O and 64.432gAl(NO 3 ) 3 9H 2 O, add 687.0ml of deionized water to prepare solution #1; weigh 87.941g NaOH and 14.564g NaOH 2 CO 3 , adding 2336.0ml of deionized water to prepare solution #2; under the conditions of 78°C and pH=10.0±0.5, carry out co-precipitation reaction between solutions #1 and #2, and continue to stir and age for 18 hours; after aging, The mixed solution was filtered and washed three times, and the obtained precipitate was transferred to a vacuum drying oven, dried at 105°C for 12 hours, and then calcined at 700°C for 4 hours to obtain the CUT-ZNA catalyst. The molar composition of the catalyst is (ZnO) 2.4 (NiO) 0.6 (AlO 1.5 ) 1 , The weight percent composition is: nickel oxide 15.4%, zinc oxide 67.1%, aluminum oxide 17.5%.
[0025] The reactivity evaluation of autothermal reforming was carried out in a continuous flow fixed bed reactor. Grind, tablet, crush, and sieve the...
example 2
[0028] Weigh 29.386gNi(NO 3 ) 2 ·6H 2 O, 120.247gZn(NO 3 ) 2 ·6H 2 O, 50.543g Al(NO 3 ) 3 9H 2 O and 13.608gFe(NO 3 ) 3 9H 2 O, add 674.0ml of deionized water to make solution #1; weigh 86.231gNaOH and 14.281gNaOH 2 CO 3 , adding 2290.0ml of deionized water to prepare solution #2; the follow-up steps were the same as in Reference Example 1, and CUT-ZNA was obtained 0.8 f 0.2 catalyst. The molar composition of the catalyst is (ZnO) 2.4 (NiO) 0.6 (AlO 1.5 ) 0.8 (FeO 1.5 ) 0.2 , The weight percent composition is: 15.1% of nickel oxide, 65.8% of zinc oxide, 13.7% of aluminum oxide, and 5.4% of iron oxide.
[0029] The CUT-ZNA 0.8 f 0.2 The activity of the catalyst was tested by autothermal reforming of acetic acid, the reduction temperature was 700°C, the reaction conditions were 650°C, CH 3 COOH / H 2 O / O 2 / N 2 =1 / 4 / 0.28 / 3.9, normal pressure, space velocity 11250ml g -1 h -1 , The reaction time is 10h. The acetic acid conversion rate of the catalyst dr...
Embodiment 1
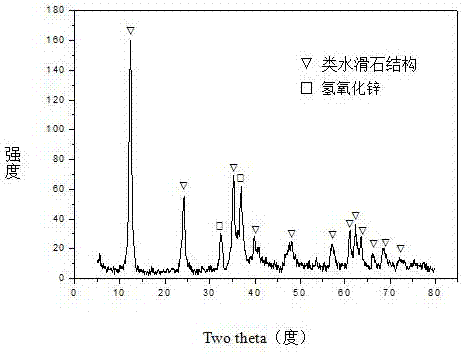
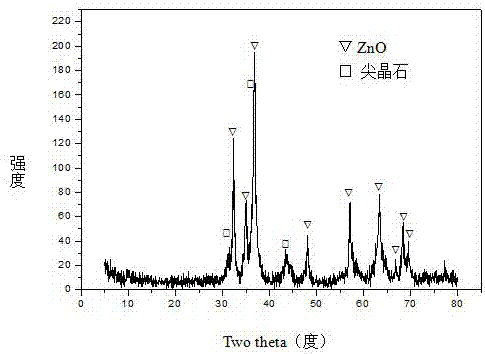
[0031] Weigh 28.553g Ni(NO 3 ) 2 ·6H 2 O, 116.839gZn (NO 3 ) 2 ·6H 2 O, 30.694g Al(NO 3 ) 3 9H 2 O and 33.055gFe(NO 3 ) 3 9H 2 O, add 655.0ml of deionized water to prepare solution #1; weigh 83.786g NaOH and 13.876g NaOH 2 CO 3 , adding 2226.0ml of deionized water to prepare solution #2; the follow-up steps are the same as those in Reference Example 1, and a precursor with a hydrotalcite-like structure as the main body and a small amount of zinc hydroxide phase is obtained, as shown in the attached figure 1 As shown, the zinc oxide-containing spinel phase (NiAl 2 o 4 / NiFe 2 o 4 / Fe 3 o 4 / ZnAl 2 o 4 ) Zn-Ni-Al-Fe-O composite oxide, as attached figure 2 As shown, that is, get CUT-ZNA 0.5 f 0.5 catalyst. The molar composition of the catalyst is (ZnO) 2.4 (NiO) 0.6 (AlO 1.5 ) 0.5 (FeO 1.5 ) 0.5 , The weight percent composition is: 14.7% of nickel oxide, 63.9% of zinc oxide, 8.3% of aluminum oxide, and 13.1% of iron oxide.
[0032] The CUT-ZNA 0.5 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com