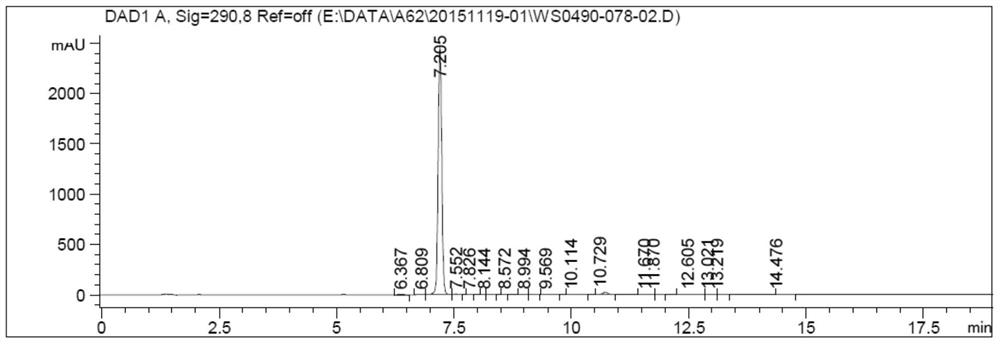
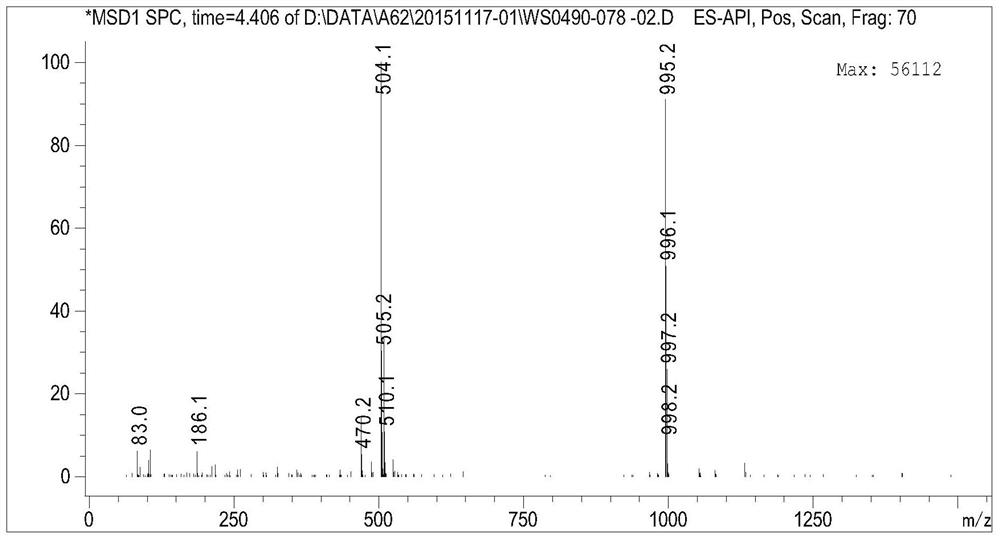
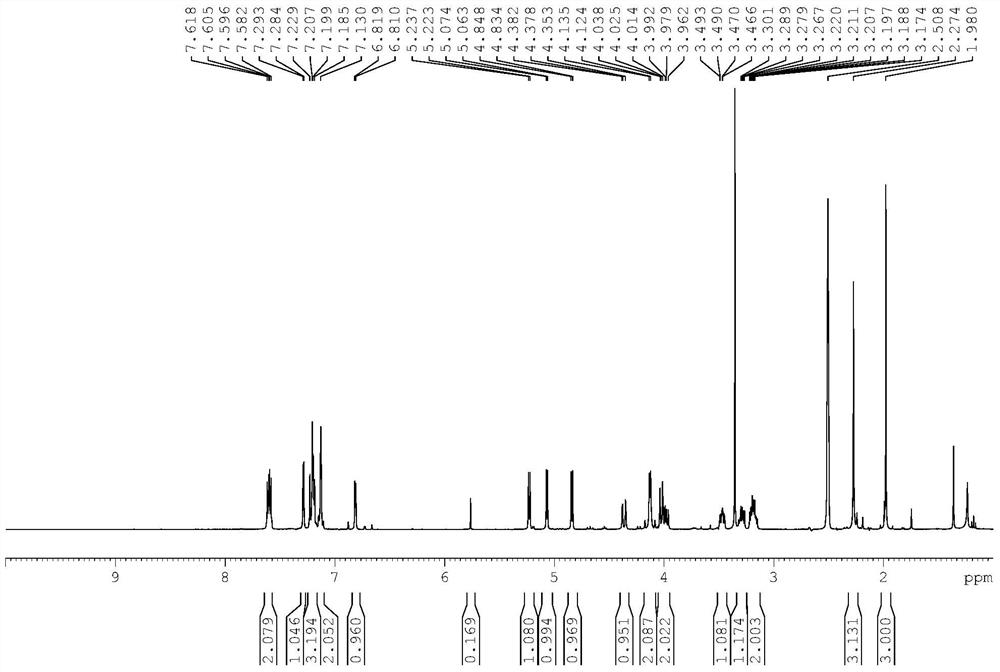
Canagliflozin drug impurity and its preparation method and application
A compound and residue technology, applied in the field of compound and its preparation, can solve the problems that the quality evaluation method of canagliflozin products needs to be improved, and achieve the effect of easy control of process operation, cheap and easy-to-obtain raw materials, and efficient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The preparation of compound shown in embodiment 1 formula 2
[0053] Step 1: Synthesis of 2,3,4,6-tetra-O-trimethylsilyl-D-gluconolactone:
[0054] Add 8.0 kg of tetrahydrofuran, 1.0 kg of gluconolactone, and 4.54 kg of N-methylmorpholine to the reaction kettle at room temperature. Under the protection of nitrogen, start to add 3.66 kg of trimethylchlorosilane dropwise. After the reaction is complete, add ice water to quench reaction. 26 kg of purified water and 5.0 kg of n-heptane were added to the reaction liquid, stirred, and allowed to stand to separate layers. Wash the organic layer with 15 kg of 5% sodium dihydrogen phosphate aqueous solution, 15 kg of purified water and 15 kg of sodium chloride aqueous solution, add anhydrous magnesium sulfate, dry, filter and concentrate under reduced pressure until there is no distillate. 2.41 kg of colorless oil was obtained, which was 2,3,4,6-tetra-O-trimethylsilyl-D-gluconolactone, with a yield of 92% and a gas phase purit...
Embodiment 2
[0061] Compound shown in embodiment 2 synthetic formula 3
[0062] Take the above solid (2,3,4,6-tetra-O-acetyl-(1S)-1,5-anhydro-1-[3-[[5-(4-fluorophenyl)-2-thienyl ]methyl]-4-methylphenyl]-D-glucitol) 1.2kg, dissolved in 8.4 liters of methanol and 6.0 liters of tetrahydrofuran at room temperature, cooled to 0 ~ 5 ℃, then dropwise added monohydrate lithium hydroxide solution (48g dissolved in 480 ml of water), then reacted at room temperature for 2 hours. Add 4.0 L of water to dilute the reaction, concentrate under reduced pressure to remove the solvent, and extract the reaction solution with ethyl acetate. Washed twice with water, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain the crude product of white foamy solid canagliflozin anhydrous (compound shown in formula 3), with a yield of 95% and a liquid phase purity of 99.2 %.
Embodiment 3
[0063] Compound shown in embodiment 3 preparation formula 1
[0064] Dissolve 800 g of the above compound in ethyl acetate, add purified water, heat up to 40 degrees Celsius, add n-heptane dropwise, add canagliflozin seed crystals, grow crystals for 3 hours and filter to obtain 135 g of mother liquor containing the compound shown in formula 1 In the concentrate, the liquid phase content of compound 1 was detected to be about 5.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com