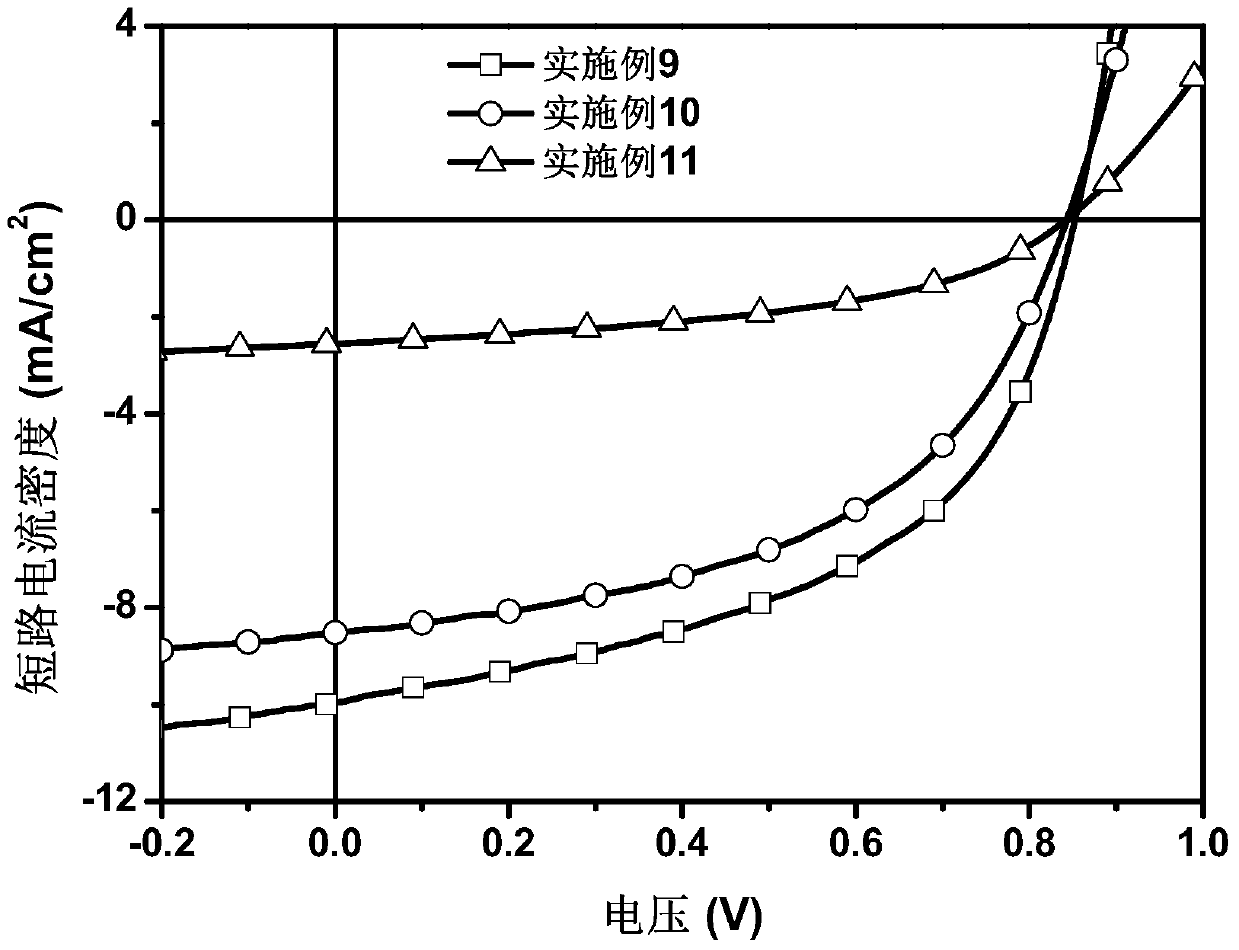
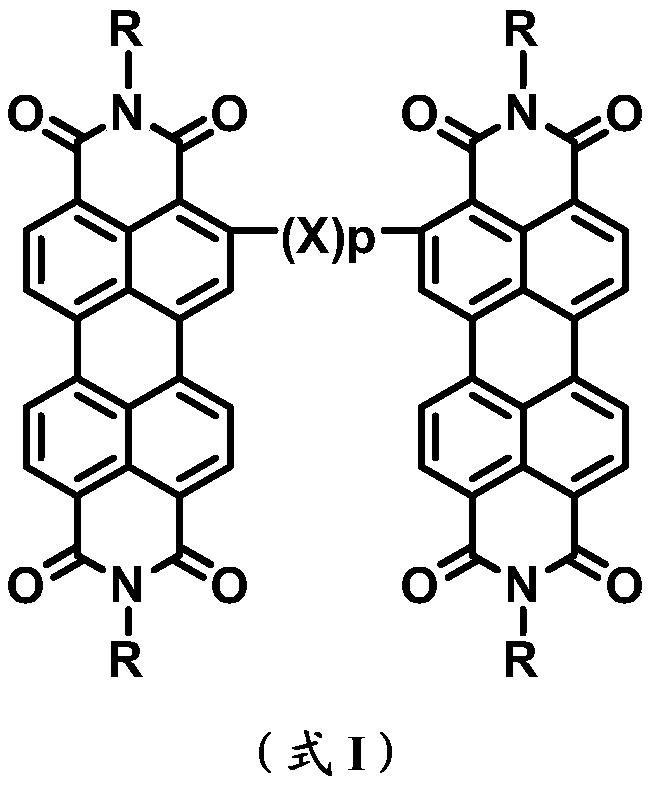
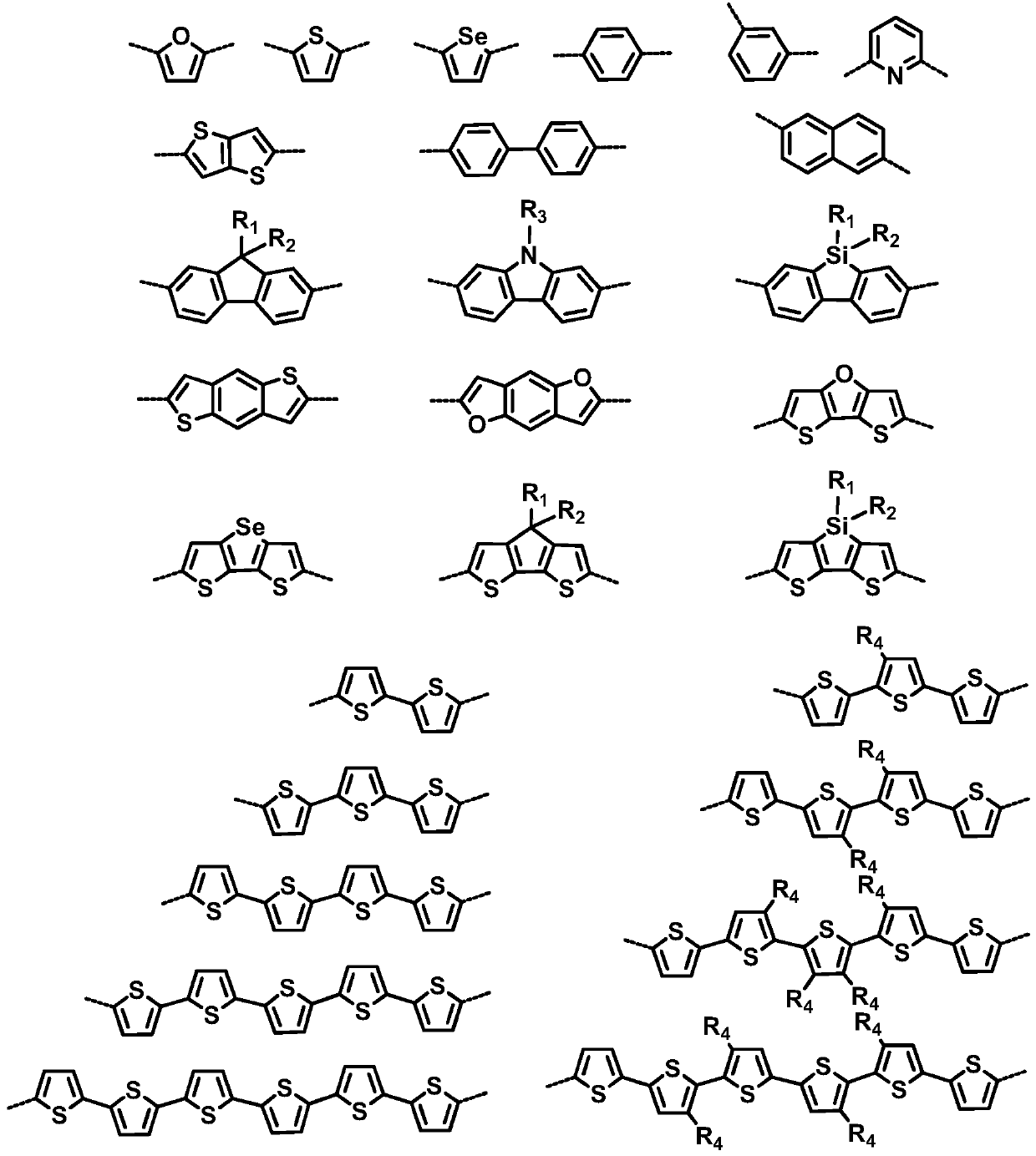
A kind of ortho-bridged perylene diimide dimer and its preparation method and application in organic photovoltaic devices
A dimer, ortho-position technology, applied in the fields of electrical solid device, semiconductor device, semiconductor/solid-state device manufacturing, etc., can solve the problems of destroying the order of PDI molecular π system, deformation, unfavorable carrier effective transport, etc. Achieve the effect of reducing excessive aggregation, reducing size, and avoiding deformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: Preparation of PDI monomer 1a substituted by ortho-position Br
[0058]
[0059] Ortho Br-substituted PDI monomer 1a according to the literature (K.Müllen et al, Org. Lett., 2011, 13, 3012-3015; H.Shinokubo et al, Org. Lett., 2011, 13, 2532-2535) method of preparation.
Embodiment 2
[0060] Embodiment 2: preparation compound 2a
[0061]
[0062] The product 1a (855.6 mg, 1.1 mmol) was dissolved in 15 mL of toluene, and commercially available thiophene-2,5-di-tributyltin (332.1 mg, 0.5 mmol) and Pd (PPh 3 ) 4 (34.7mg, 0.03mmol), under the heating condition of 120°C, the reaction was stirred for 36 hours. The toluene in the reaction system was distilled off under reduced pressure to obtain a red-black solid, which was dissolved in a mixed solvent of 50 mL of dichloromethane and 50 mL of water, the dichloromethane layer was collected by liquid separation, and the dichloromethane was distilled off under reduced pressure to obtain a red The black solid was passed through the H60 silica gel column to obtain the product 2a (628.2 mg, 0.425 mmol, yield: 85%). 1 H NMR (400MHz, CDCl 3 ):δppm:8.68-8.62(m,14H),7.49-7.37(m,2H),5.21-5.13(m,4H),2.28-2.21(m,8H),1.87-1.80(m,8H),1.36 -1.15(m,48H),1.14-0.96(m,24H). MS(MALDI-TOF):m / z=1477.2[M+H] + .
Embodiment 3
[0063] Example 3: Preparation of Trimethyltin Derivatives Used in Examples 4-7
[0064] Oligothiophene was synthesized according to the literature (N.Tamaoki et al, J.Am.Chem.Soc., 2006, 128, 10930-10933).
[0065] The trimethylstanning reaction was prepared as follows: oligothiophene, selenophene, benzene, or furan (1 mmol) was dissolved in 10 mL of anhydrous THF. Argon protection, cooled to -78 ℃. A commercially available 2.2 mol / L n-butyllithium solution in n-hexane (1 mL, 2.2 mmol) was slowly added. After stirring at -78°C for 2 hours, a commercially available 1 mol / L (25 wt.%) trimethyltin chloride solution in n-hexane (2.5 mL, 2.5 mmol) was added. After the reaction system was warmed to room temperature, stirring was continued for 2 hours. The mixed solution was diluted with 50 mL of ethyl acetate. After washing three times with water, dry and spin dry. The residue was recrystallized with methanol at -20°C, and the corresponding trimethyltin derivatives could be sep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com