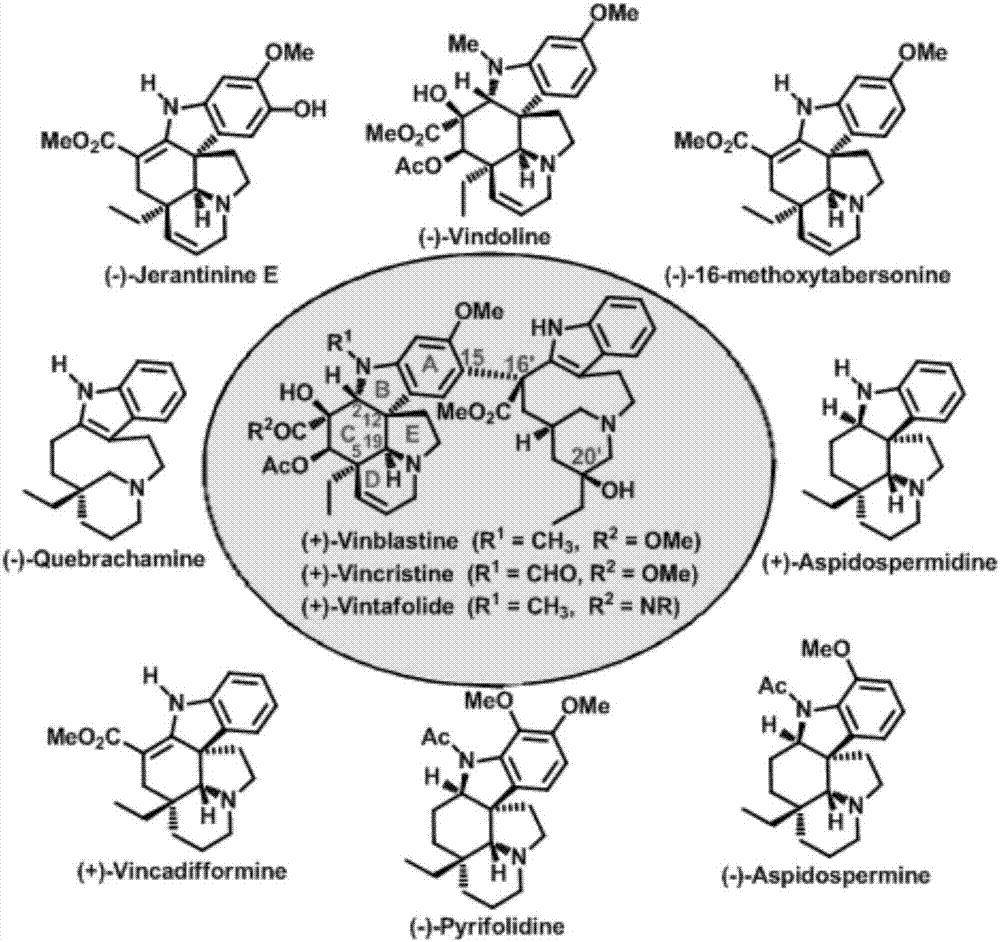
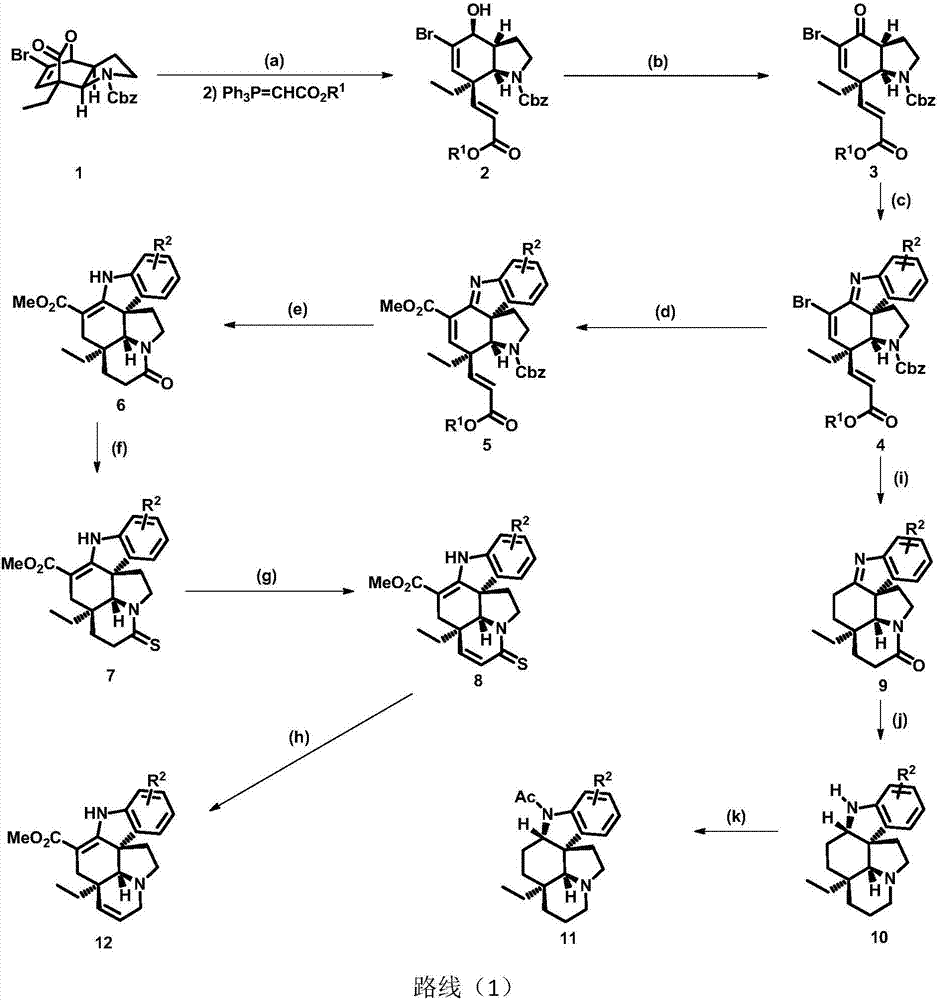
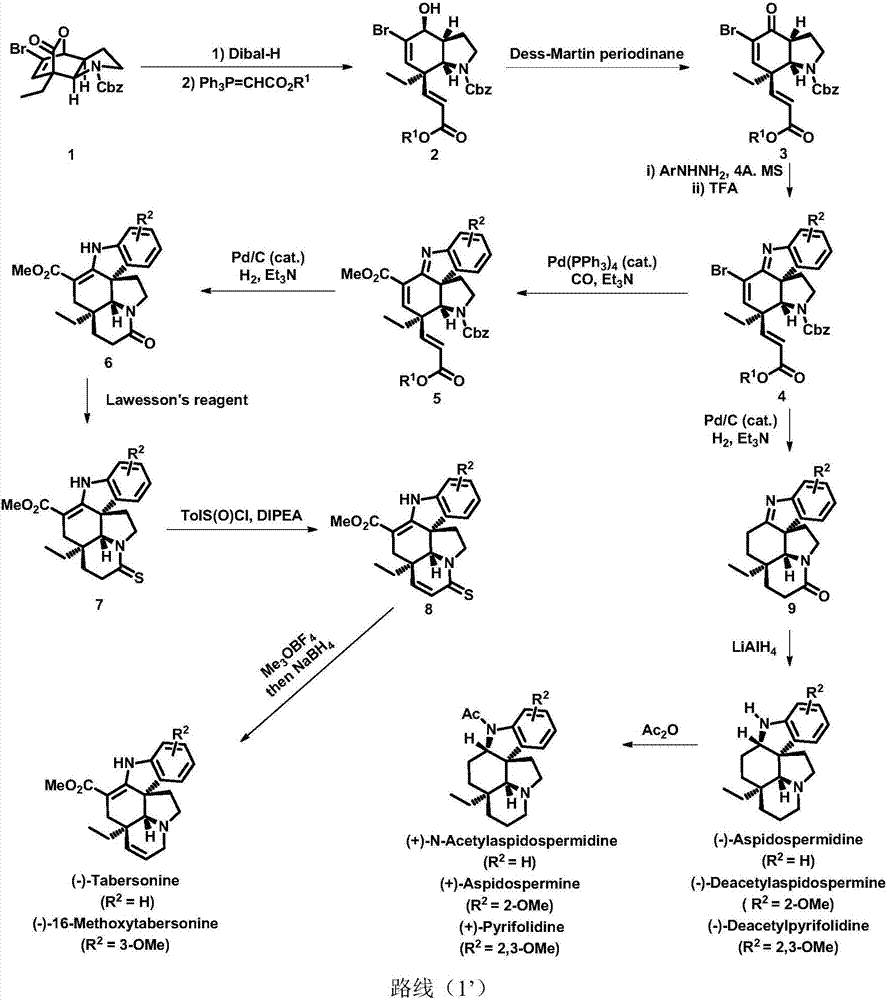
Method for asymmetric synthesis of Aspidosperma alkaloids
An alkaloid and asymmetric technology, applied in asymmetric synthesis, organic chemistry methods, chemical instruments and methods, etc., can solve chiral synthesis with low abundance and inability to achieve structural diversity, single achiral natural products, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] Synthesis of formula (2a) compound
[0119]
[0120] The compound of formula (1) (4.09g, 2.61mmol, 1.0equiv.) was dissolved in toluene (100mL), and diisobutylaluminum hydride (10mL, 3.91mmol, 1.5equiv., 1.5Mintoluene) The reaction was raised to -40°C and stirred for 3 hours. The reaction was quenched with saturated sodium potassium tartrate solution (20 mL), and stirred at room temperature until the reaction solution was clear. The toluene was removed under reduced pressure, and the aqueous phase was extracted with ethyl acetate (3x50 mL), dried over anhydrous sodium sulfate, filtered, and the solvent was removed under reduced pressure to obtain a colorless oily liquid (no need for separation and purification by column chromatography).
[0121] Dissolve this oily liquid in toluene (100mL), add Ph 3 P=CHCO 2 Me (5.05g, 3.41mmol, 1.5equiv.), the reaction was raised to reflux at 110°C for 4 hours. After removing toluene under reduced pressure, direct column chromato...
Embodiment 2
[0124] Synthesis of formula (3a) compound
[0125]
[0126] The compound of formula (2a) (3.55g, 7.65mmol, 1.0equiv.) was dissolved in dichloromethane (76mL), and Dess-Martin oxidant (3.89g, 9.17mmol, 1.2equiv.) was added at 0°C to react Warm to room temperature and stir for 1 hour. After completion of the reaction, add saturated sodium thiosulfate solution (25mL) and saturated sodium bicarbonate solution (25mL) successively, extract the system with dichloromethane (3x50mL) and dry over anhydrous sodium sulfate, filter, remove the solvent under reduced pressure, and perform column chromatography Isolation (petroleum ether: ethyl acetate = 10:1) gave white foam formula (3a) compound (3.23g, 92%).
[0127] The detection data of formula (3a) compound is as follows: 1 HNMR (500MHz, CDCl 3 )δ7.38–7.35(m,5H),7.15(s,1H),6.99(d,J=16.2Hz,1H),5.73(d,J=16.2Hz,1H),5.19(s,1H), 5.14–5.10(m,1H),4.70(d,J=7.8Hz,1H),3.73(s,3H),3.61–3.53(m,1H),3.47–3.39(m,1H),3.09(dt, J=12.8,8.3Hz,1H),2.35...
Embodiment 3
[0129] Synthesis of formula (4a) compound
[0130]
[0131] Formula (3a) (3.23g, 7.01mmol, 1.0equiv), 4A, MS (1.26g), sodium carbonate (2.45g, 14.02mmol, 2.0equiv.) and 3-methoxyphenylhydrazine (1.48g, 14.02 mmol, 2.0 equiv.) was dissolved in toluene (140 mL), and the reaction was raised to 110° C. and refluxed for 12 hours. Sodium carbonate (2.45 g, 14.02 mmol, 2.0 equiv.) and 3-methoxyphenylhydrazine (1.48 g, 14.02 mmol, 2.0 equiv.) were added. After the reaction was completed, it was cooled to room temperature, and the benzene was removed under reduced pressure to obtain a brown solid directly (without separation and purification by column chromatography). The brown solid was dissolved in 1,2-dichloroethane (140 mL), and the reaction was raised to 80° C. and trifluoroacetic acid (15.96 g, 140.2 mmol, 20.0 equiv) was added to reflux for 1 hour. After the reaction was completed, cool to room temperature, adjust the pH to 8-9 with saturated sodium bicarbonate solution, ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com