Oral nilotinib nano-formulation and preparation method thereof
A technology of nilotinib and nano-preparation, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of low solubility and low bioavailability, and achieve product quality. High, improve drug solubility and permeability, the effect of continuous operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A kind of preparation of nilotinib oral nano-preparation, the steps are as follows:
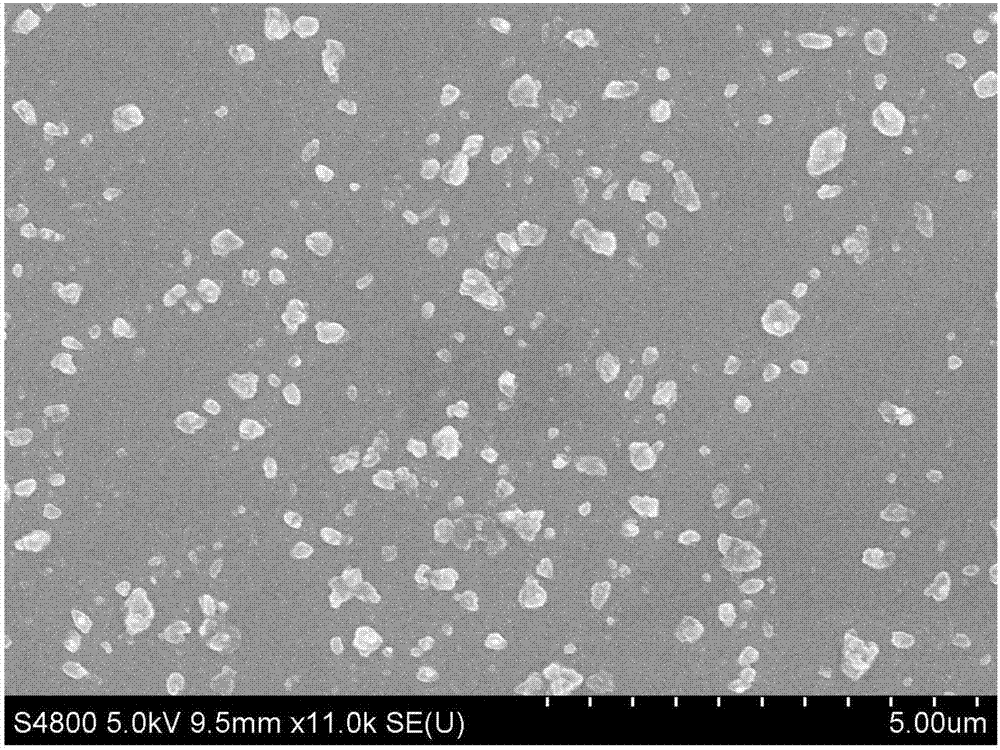
[0044] Nilotinib bulk drug (scanning electron microscope picture as figure 1 shown) and N,N-dimethylformamide to make 40mL of nilotinib N,N-dimethylformamide solution with a concentration of 15mg / mL; make 0.2mg of natural water-soluble VE and deionized water 600mL of natural water-soluble VE aqueous solution per mL, add 20mg sodium dodecylbenzenesulfonate to the natural water-soluble VE aqueous solution at the same time to obtain an anti-solvent containing a stabilizer, then add nilotinib solution, and stir for 10min using a mechanical stirrer , the temperature of the reaction system is controlled to be 15°C to obtain a nilotinib drug slurry; the obtained nilotinib drug slurry is freeze-dried to obtain a freeze-dried powder, and the obtained freeze-dried powder is mixed with 400mg lactose, 20mg cross-linked carboxyl Sodium methylcellulose, 15mg talcum powder, and 15mg silicon dioxide ...
Embodiment 2
[0051] Nilotinib raw material drug and N,N-dimethylformamide are made into 20mL of nilotinib N,N-dimethylformamide solution with a concentration of 10mg / mL; into 0.1mg / mL polyvinylpyrrolidone aqueous solution 300mL, add 5mg poloxamer to polyvinylpyrrolidone aqueous solution simultaneously, obtain the anti-solvent containing stabilizer; Adjust the flow rate to be 2mL / min, 30mL / min respectively, collect the product at the outlet of the microchannel reactor, and control the temperature of the reaction system to obtain the nilotinib nano-medicine slurry; the obtained nilotinib nano-medicine slurry The material is freeze-dried to obtain freeze-dried powder; the obtained freeze-dried powder is mixed with 150mg lactose, 10mg crospovidone, 5mg magnesium stearate, 5mg colloidal silicon dioxide, and 5mg talcum powder to prepare nilotinib for oral administration. nano formulations.
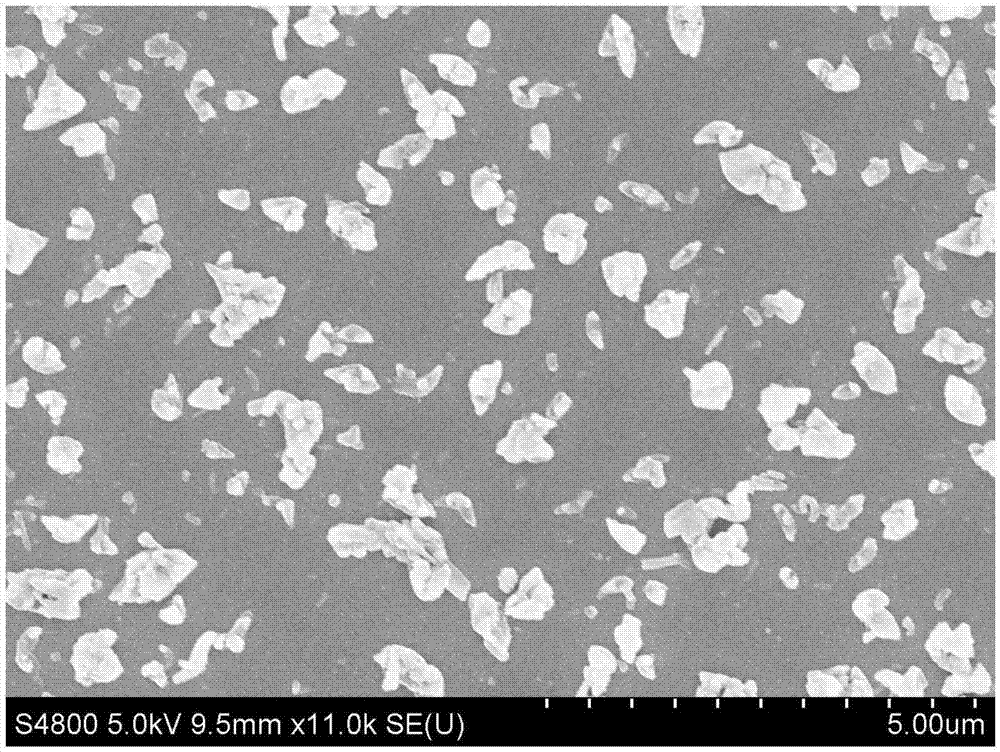
[0052] The obtained freeze-dried powder is redispersed with water, and the SEM characterization shows th...
Embodiment 3
[0054] The nilotinib bulk drug and N,N-dimethylformamide are formulated into 40mL of nilotinib N,N-dimethylformamide solution with a concentration of 15mg / mL; polyethylene glycol and deionized water Prepare 800 mL of 0.1 mg / mL polyethylene glycol solution with ethanol, wherein the volume ratio of alcohol to water is 1:5, and simultaneously add 20 mg of sodium dodecylbenzenesulfonate and 50 mg of lactose to the PEG solution to obtain polyethylene glycol containing Anti-solvent of alcohol, sodium dodecylbenzenesulfonate and lactose; Nilotinib solution was added to the anti-solvent, stirred with a magnetic stirrer for 5min, and the temperature of the reaction system was controlled at 20°C to obtain Nilotinib nano Drug slurry; the obtained nilotinib nano drug slurry is spray-dried to obtain a spray-dried powder; the gained spray-dried powder is mixed with 350mg lactose, 60mg crospovidone, 15mg magnesium stearate, and 15mg silicon dioxide to prepare Oral nano-formulations of niloti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com