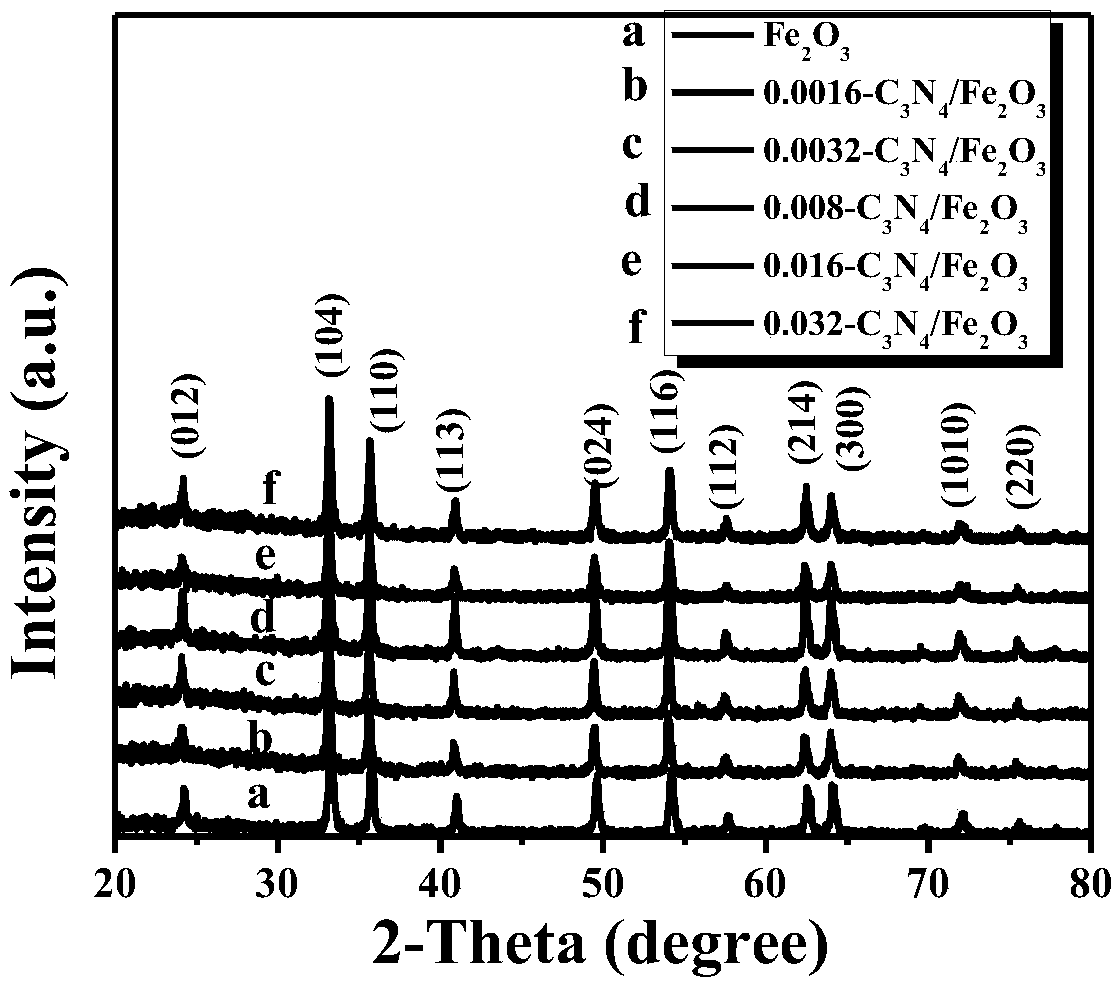
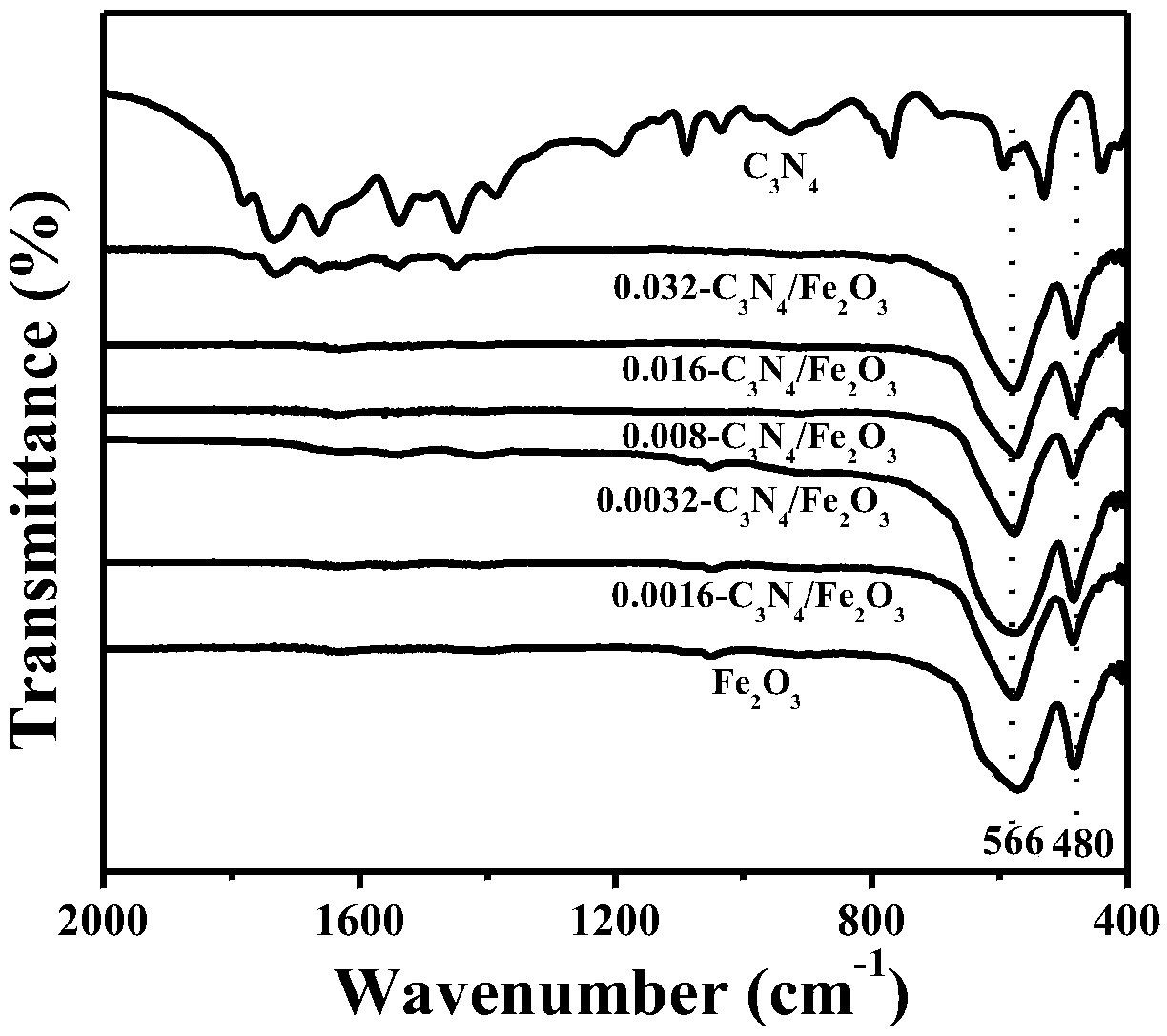
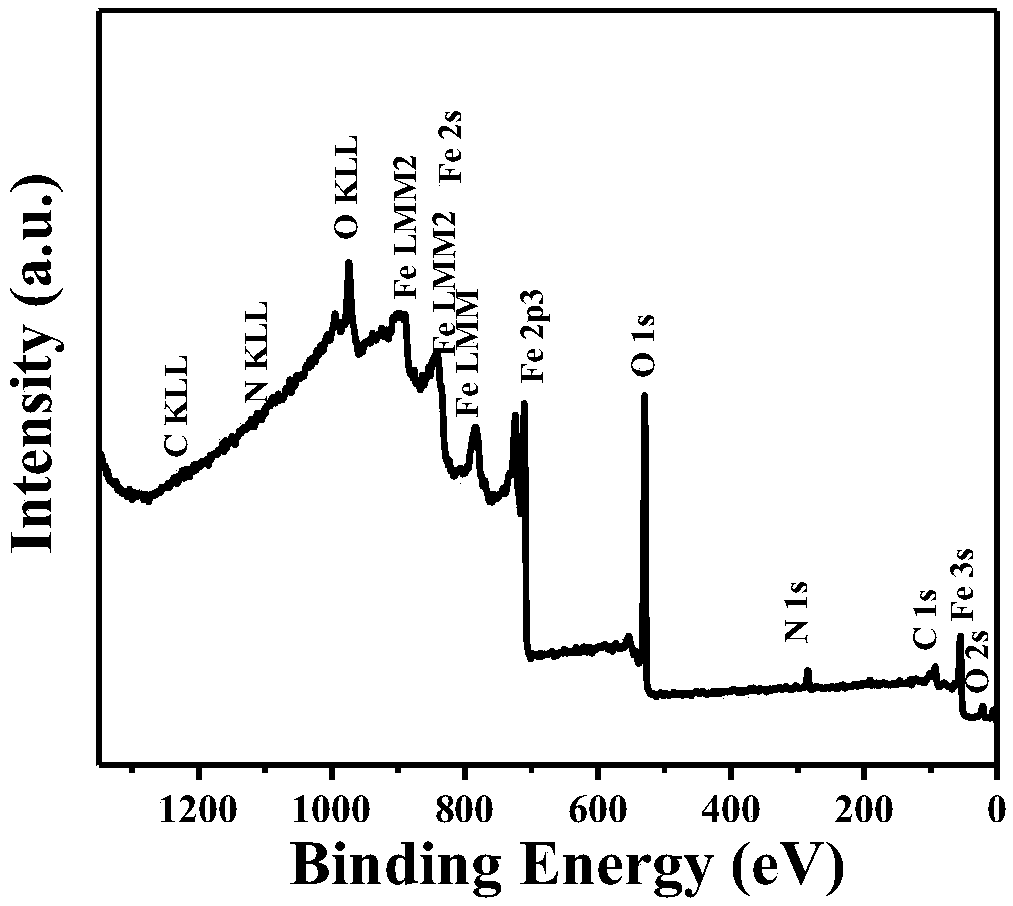
A preparation method based on iron oxide doped graphite phase carbon nitride composite material
A graphite phase carbon nitride and composite material technology, applied in the direction of iron oxide/iron hydroxide, iron oxide, nitrogen compounds, etc., can solve the problems of easy agglomeration of active sites, high electron-hole pair recombination rate, and reduce the Effect of recombination efficiency, facile preparation process, and excellent photoelectrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Preparation of iron-based ionic liquid: at room temperature, weigh 20g T 8 Cl (trioctylmethylammonium chloride) was stirred in a 50mL round bottom flask, and 13.376g FeCl was added 3 ·6H 2 O. After stirring evenly, the three-neck flask was placed in an oil bath at 40°C, and stirred for 24 hours. After the reaction was completed, the reactant was filtered and then dried in a blast oven at 50° C. for 24 hours. The prepared iron-based ionic liquid T 8 Cl / FeCl 3 Sealed and kept for later use.
[0028] (2) Weigh 5 mmol of cyanuric chloride, add it into a polytetrafluoroethylene-lined reaction kettle, then measure 20 mL of acetonitrile and add it into the reaction kettle, stir magnetically at room temperature for a certain period of time until the cyanuric chloride dissolves. Finally, 2.5 mmol of melamine was weighed and slowly added to the reaction kettle. After magnetic stirring at room temperature for 15 minutes, the reaction was performed in a high-temperature s...
Embodiment 2
[0034] (1) Preparation of iron-based ionic liquid: at room temperature, weigh 15g T 8 Cl (trioctylmethylammonium chloride) was stirred in a 50mL round bottom flask, and 10.032g FeCl was added 3 ·6H 2 O. After stirring evenly, the three-neck flask was placed in an oil bath at 40°C, and stirred for 24 hours. After the reaction was completed, the reactant was filtered and then dried in a blast oven at 50° C. for 24 hours. The prepared iron-based ionic liquid T 8 Cl / FeCl 3 Sealed and kept for later use.
[0035] (2) Weigh 3.5 mmol of cyanuric chloride, add it into a polytetrafluoroethylene-lined reaction kettle, then measure 20 mL of acetonitrile and add it into the reaction kettle, stir magnetically at room temperature for a certain period of time until the cyanuric chloride dissolves. Finally, 1.75 mmol of melamine was weighed and slowly added to the reaction kettle. After magnetic stirring at room temperature for 15 minutes, the reaction was performed in a high-temperatur...
Embodiment 3
[0041] (1) Preparation of iron-based ionic liquid: at room temperature, weigh 10g T 8 Cl (trioctylmethylammonium chloride) was stirred in a 50mL round bottom flask, and 6.688g FeCl was added 3 ·6H 2 O. After stirring evenly, the three-neck flask was placed in an oil bath at 40°C, and stirred for 24 hours. After the reaction was completed, the reactant was filtered and then dried in a blast oven at 50° C. for 24 hours. The prepared iron-based ionic liquid T 8 Cl / FeCl 3 Sealed and kept for later use.
[0042] (2) Weigh 2.5mmol of cyanuric chloride, add it into a polytetrafluoroethylene-lined reaction kettle, then measure 20mL of acetonitrile into the reaction kettle, stir magnetically at room temperature for a certain period of time until the cyanuric chloride dissolves. Finally, 1 mmol of melamine was weighed and slowly added to the reaction kettle. After magnetic stirring at room temperature for 15 minutes, the reaction was performed in a high-temperature solvent heating...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com