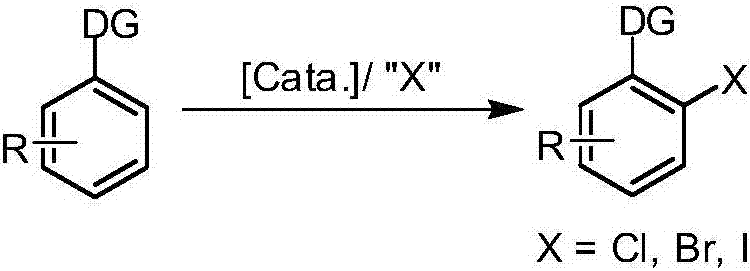
Synthetic method of aryl halide taking aryl carboxylic acid as raw material
A technology of aryl halides and synthesis methods, which is applied in the field of synthesis and preparation of chemical products, can solve the problems of large amount of cuprous salt, etc., and achieve the effects of small amount, good tolerance, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1. Synthesis of 2-nitro-4-chloroiodobenzene.
[0054] Add 6.2 mg of silver sulfate, 21.8 mg of copper acetate, 12.5 mg of 2,9-dimethyl-1,10-phenanthroline, 2-nitro-4-chloro Benzoic acid 40.3 mg, sodium iodide 45 mg and 4 mL of dimethyl sulfoxide. Heated at 160°C for 24 hours in the presence of oxygen. After the reaction was completed, distilled water was added to quench the reaction, and ethyl acetate was used to extract 3 times, 10 mL each time, and the combined organic phase was concentrated to obtain 40.8 mg of 2-nitro-4-chloroiodobenzene, with a yield of 72%. .
[0055] Product 2-nitro-4-chloroiodobenzene: 1 H NMR (400MHz, CDCl 3 ): δ7.95(d, J=8.5Hz, 1H), 7.84(d, J=2.0Hz, 1H), 7.28-7.25(m, 1H); 13 C NMR (100MHz, CDCl 3 ): δ142.7, 135.3, 133.6, 125.7, 83.6.
Embodiment 2
[0056] Example 2. Synthesis of 2-nitro-4-chlorobromobenzene.
[0057] Add 6.2 mg of silver sulfate, 36.3 mg of copper acetate, 12.5 mg of 2,9-dimethyl-1,10-phenanthroline, 2-nitro-4-chloro Benzoic acid 40.3 mg, sodium bromide 30.9 mg and 4 mL of dimethyl sulfoxide. Heated at 160°C for 24 hours in the presence of oxygen. After the reaction was completed, distilled water was added to quench the reaction, and ethyl acetate was used to extract 3 times, 10 mL each time, and the combined organic phase was concentrated to obtain 30.3 mg of 2-nitro-4-chlorobromobenzene, with a yield of 64%. .
[0058] Product 2-nitro-4-chlorobromobenzene: 1 H NMR (400MHz, CDCl 3 ): δ7.85(d, J=2.1Hz, 1H), 7.69(d, J=8.6Hz, 1H), 7.42(dd, J=2.2, 8.5Hz, 1H). 13 C NMR (100MHz, CDCl 3 ): δ135.9, 133.3, 125.8, 112.6.
Embodiment 3
[0059] Example 3. Synthesis of 2-nitro-1,4-dichlorobenzene.
[0060] Add 6.2 mg of silver sulfate, 36.3 mg of copper acetate, 12.5 mg of 2,9-dimethyl-1,10-phenanthroline, 2-nitro-4-chloro Benzoic acid 40.3 mg, sodium chloride 17.5 mg and 4 mL of dimethyl sulfoxide. Heated at 160°C for 24 hours in the presence of oxygen. After the reaction was completed, distilled water was added to quench the reaction, and ethyl acetate was used to extract 3 times, 10 mL each time, and the combined organic phase was concentrated to obtain 20.7 mg of 2-nitro-1,4-dichlorobenzene. The yield was 54%.
[0061] Product 2-nitro-1,4-dichlorobenzene: 1 H NMR (400MHz, CDCl3): δ7.88(s, 1H), 7.50(d, J=0.8Hz, 2H). 13 C NMR (100MHz, CDCl 3 ): δ133.3, 132.8, 125.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com