Fibroblast growth factor liposome lyophilized powder for preventing and treating alopecia, and preparation method thereof
A fibroblast and growth factor technology, applied in the field of fibroblast growth factor liposome freeze-dried powder and its preparation, can solve problems such as inability to guarantee stability, and achieves increased hair follicle drug distribution, improved transdermal absorption capacity, The effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0047] Example 13: Liposome Characterization
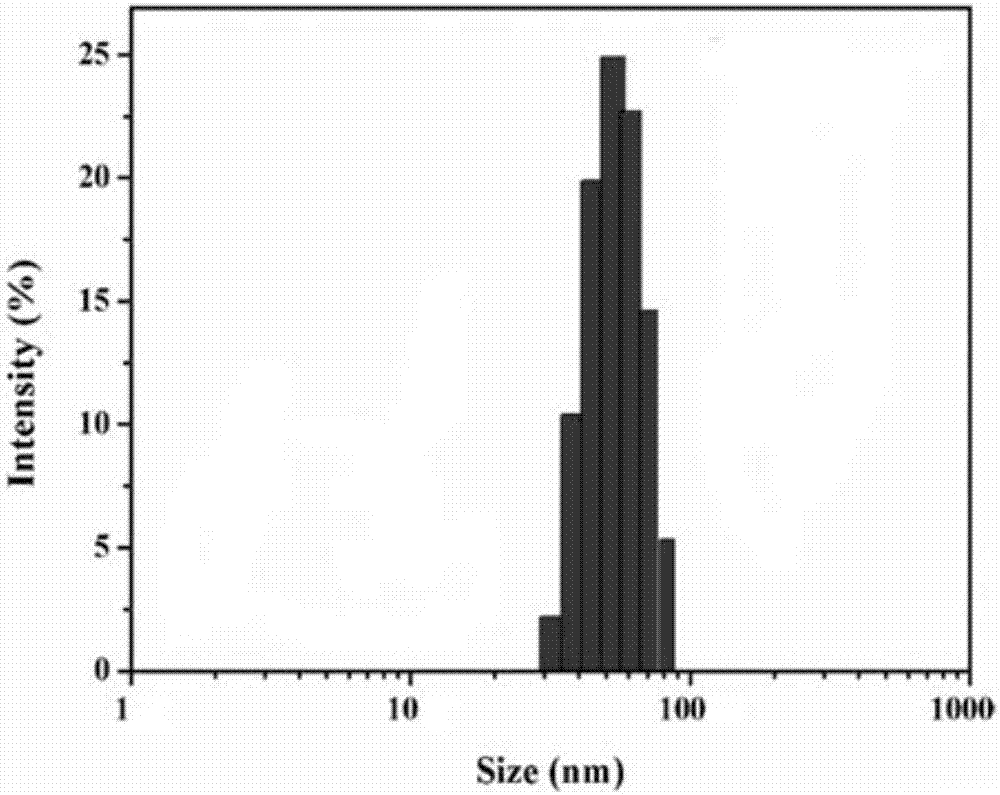
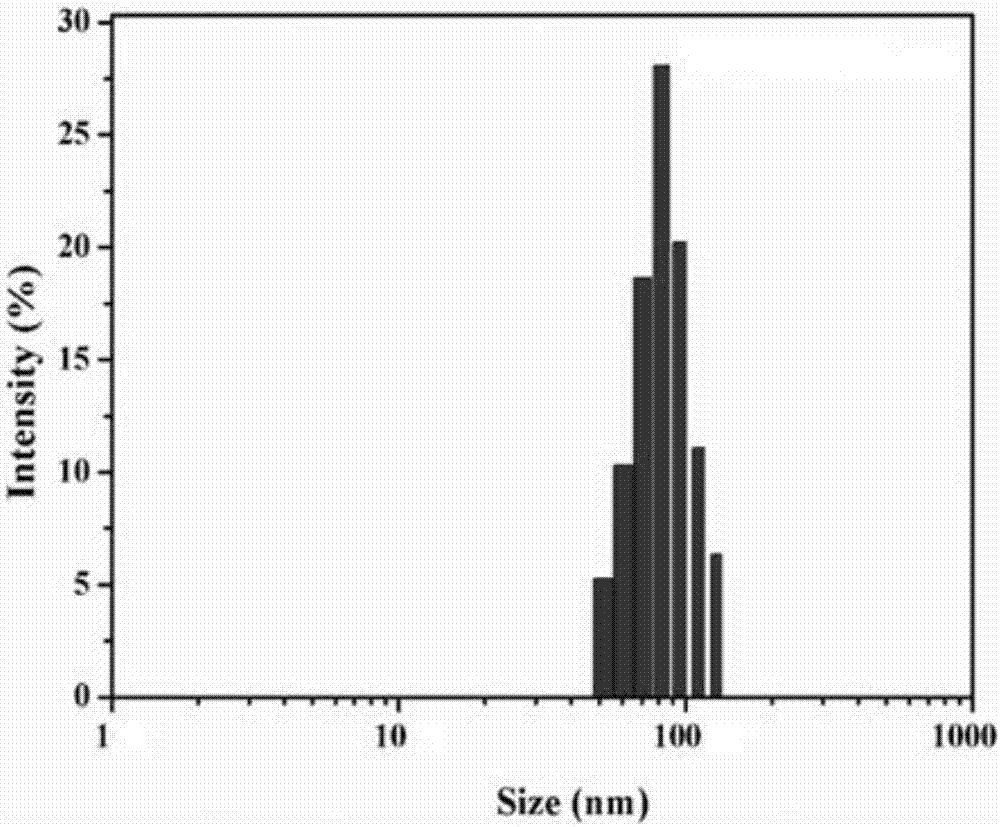
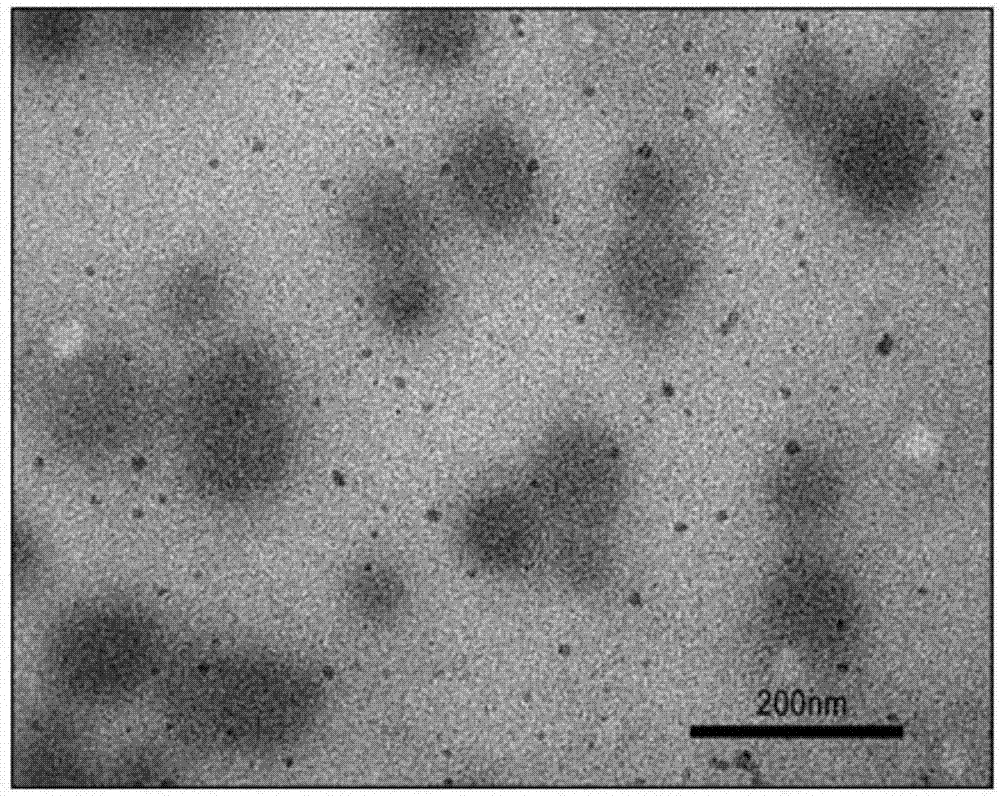
[0048] (1) Particle size measurement: After the finished products prepared in Examples 1 to 12 and the finished products prepared in comparative examples were dispersed in 10mM PBS, respectively, the particle size and particle size were measured by NICOMP 380 particle size analyzer and JEM-2000 transmission electron microscope respectively. Form and particle size results are shown in Table 2 and attached Figure 1a , 1b And attached Figure 2a , 2b shown. Visible, the particle diameter of prepared liposome finished product is between 99.5nm~800nm, wherein the finished product (particle diameter is 95.5nm) prepared by comparative example, the finished product prepared by embodiment 1 has larger particle diameter, and its particle diameter is 99.8nm, transmission electron microscopy showed an obvious gel core structure. The weight ratio of phospholipid to cholesterol has an important influence on maintaining the spherical shape ...
Embodiment 14
[0052] Example 14: Liposome Stability
[0053] The MTT method was used to detect the effect on the proliferation of HaCaT cells before and after being placed at 37°C for 2 weeks, and the percentage of liposome preparation in vitro activity was evaluated. Among them, the HaCaT cell culture medium is prepared by volume ratio of 89% RPMI-1640 medium, 10% FBS and 1% penicillin-streptomycin. 2 cultivated in an incubator. Take HaCaT cells in the logarithmic growth phase, digest with 0.25% trypsin, inoculate in a 96-well plate with about 5000 cells per well, and after incubation for 24 hours, add PBS (control sample), the finished product of Comparative Example, and the finished products of Examples 1 to 12 respectively. and bFGF solution treatment, incubated for 72 hours, the control sample was not treated, the finished products of the comparative examples, the finished products of Examples 1-12 and the bFGF solution were incubated for 72 hours, and 20 μL of 5.0 g L-1 MTT solution ...
Embodiment 15
[0054] Embodiment 15: experiment of promoting hair follicle growth in vitro
[0055] Complete rat hair follicles were selected under a dissecting microscope. The isolated complete hair follicles were cultured in a 35mm small petri dish, and the growth rate of the hair follicles cultured in vitro was divided into 5 groups, with 10 hair follicles in each group, and the experimental group had 6-7ml of conventional culture medium (containing 2ml of glutamine). The finished products of Examples 1-12 were directly dispersed in 5 ml of 10 mM phosphate buffer (pH 7.4) as the test group, and the conventional hair follicle culture solution as the negative control group. After adding 100 μL of test solution, culture in an incubator at 31°C with a certain humidity, observe the growth of hair follicles under an inverted microscope every day, take pictures and measure the growth length. After 8 days of culture, the measured hair follicle length was subtracted from the hair follicle length ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com