A crystal-type HCV inhibitor, a preparing method thereof and applications of the inhibitor
A crystalline form, isopropyl technology, applied in the field of drug development, can solve the problem that amorphous API is difficult to prepare pharmaceutical preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
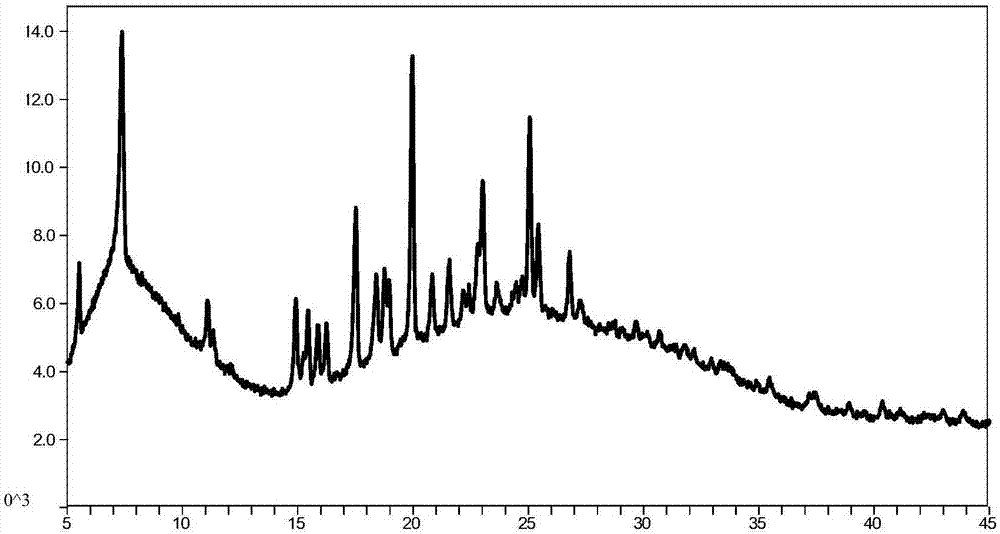
[0066] Weigh 50mg of the compound of formula I (amorphous) and place it in a 10.0mL glass bottle, then add 3.0mL of positive solvent methyl tert-butyl ether, stir to dissolve, slowly add 5.0mL of anti-solvent n-heptane, at room temperature (20- 25°C) magnetic stirring for 48 hours, solid-liquid separation to obtain the compound of crystalline formula I (crystal form I), its powder X-ray diffraction pattern is as follows figure 1 shown.
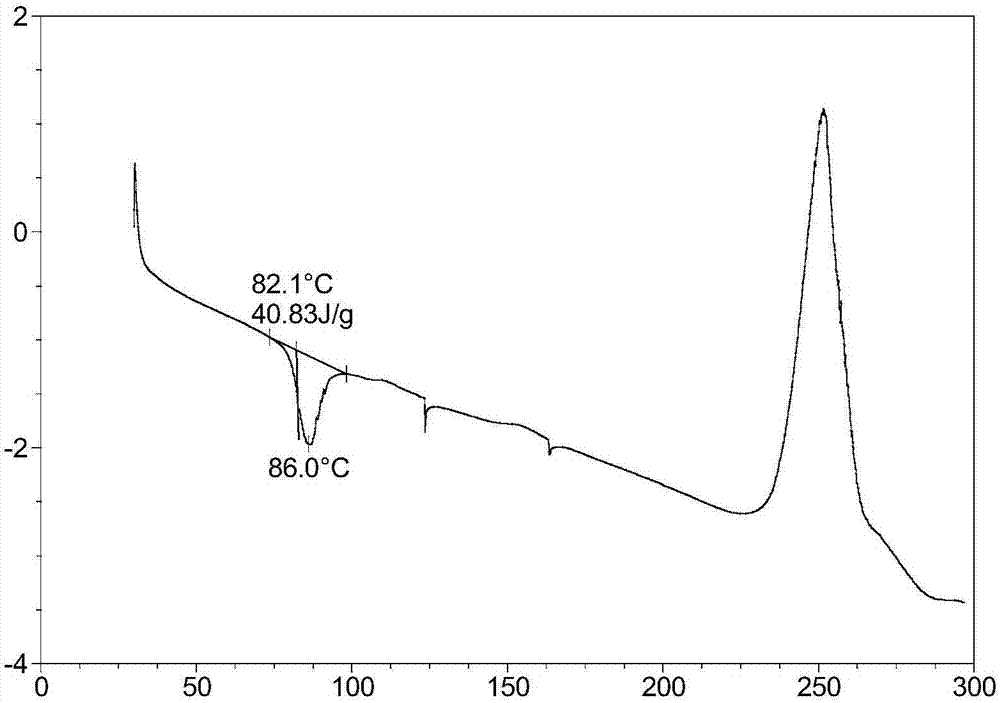
[0067] The obtained crystal form I of the compound of formula I was thermally analyzed by differential scanning calorimeter (DSC) and thermogravimetric analyzer (TGA), respectively. The DSC instrument model used was TA Q2000, and the TGA instrument model was TA Q5000. DSC, TGA analysis method parameters are as follows: the temperature range is room temperature to 300 degrees Celsius, the scan rate is 10 degrees Celsius per minute, and the protective gas is nitrogen (flow rate 25 ml / min). DSC analysis chart see image 3 , see the TGA analysis...
Embodiment 2
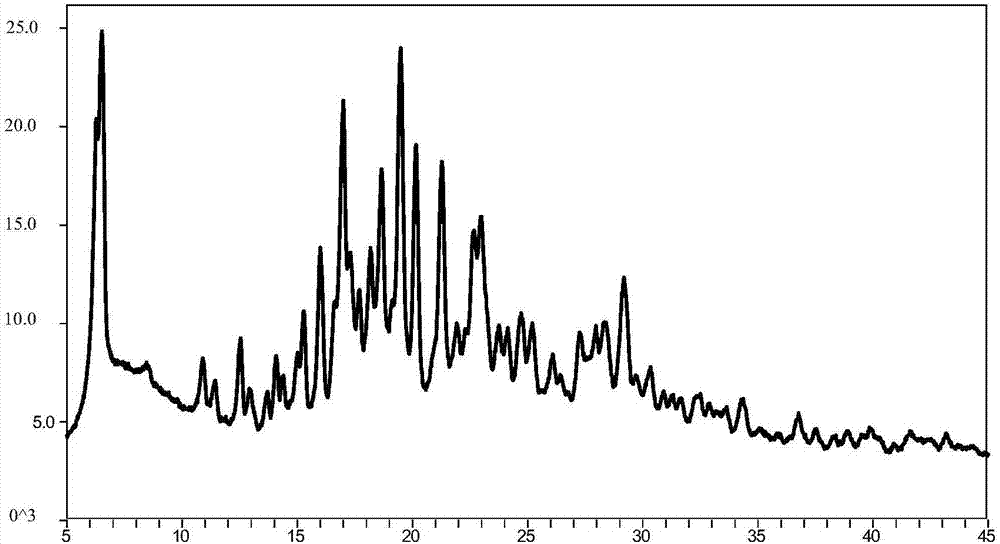
[0069] Weigh 30mg of the compound of formula I (amorphous) into a 10.0mL glass bottle, then add 2.0mL of positive solvent ethyl acetate, stir to dissolve, slowly add 5.0mL of anti-solvent n-heptane, at room temperature (20-25°C) Magnetic stirring for 48 hours, solid-liquid separation to obtain crystalline formula I compound (crystal form II), its powder X-ray diffraction pattern is as follows figure 2 shown.
[0070] The obtained crystalline form II of the compound of formula I was thermally analyzed by differential scanning calorimeter (DSC) and thermogravimetric analyzer (TGA). The DSC instrument model used was TA Q2000, and the TGA instrument model was TA Q5000. DSC, TGA analysis method parameters are as follows: the temperature range is room temperature to 300 degrees Celsius, the scan rate is 10 degrees Celsius per minute, and the protective gas is nitrogen (flow rate 25 ml / min). DSC analysis chart see Figure 5 , see the TGA analysis chart Figure 6 .
Embodiment 3
[0072] Weigh 200mg of the compound of formula I (amorphous) into a 20.0mL glass bottle, then add 5.0mL of normal solvent isopropyl ether, stir to dissolve, slowly add 10.0mL of anti-solvent n-hexane, at room temperature (20-25°C) magnetic Stir for 48 hours, solid-liquid separation to obtain the crystalline formula I compound (crystal form I), its powder X-ray diffraction pattern is as shown in figure 1 Basically the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com