Method for preparing 1,3-cyclohexanedione
A technology of cyclohexanedione and resorcinol, which is applied in the field of preparing 1,3-cyclohexanedione with high product yield, can solve the problems of affecting product yield, increasing the total amount and difficulty of waste treatment, etc. Achieve the effect of reducing production cost, high catalytic activity and stability, and long life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] Catalyst preparation
[0025] Catalyst 1: impregnated Pd
[0026] 0.1g PdCl 2 Dissolve in 10mL of water, add 2g of dry activated carbon, stir at 50°C until the water evaporates to dryness, dry in an oven at 80°C for 12h, and roast at 450°C for 5h under a nitrogen atmosphere to obtain a 3% Pd / C catalyst.
[0027] Catalyst 2: Co impregnated first and then Pd
[0028] 0.1g Co(NO 3 ) 2 ·6H 2 Dissolve O in 10 mL of water, add 2 g of dry activated carbon, stir at 50 °C until the water evaporates to dryness, dry in an oven at 80 °C for 12 h, and roast at 450 °C for 5 h under a nitrogen atmosphere to obtain a catalyst impregnated with Co, which is ready for use. 0.1gPdCl 2 Dissolve in 10 mL of water, add the catalyst impregnated with Co, stir at 50°C until the water evaporates to dryness, dry in an oven at 80°C for 12 hours, and bake at 450°C for 5 hours under a nitrogen atmosphere to obtain a 1% Co-modified Pd / C catalyst.
[0029] Catalyst 3: impregnating Pd first and t...
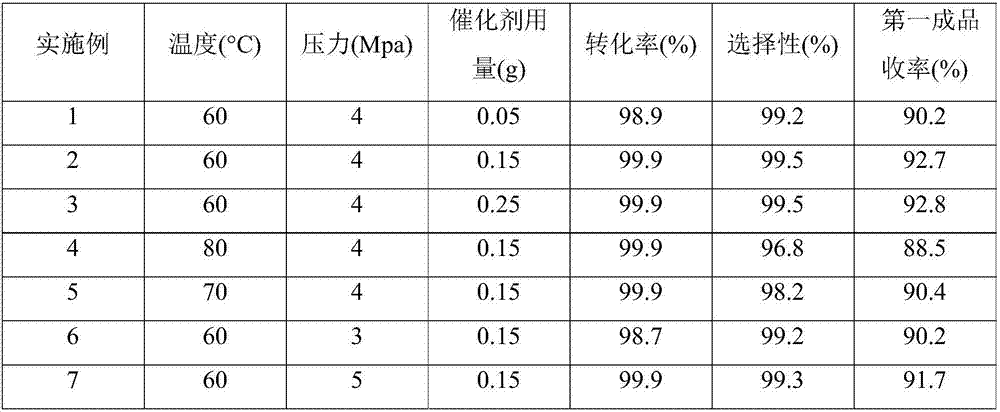
Embodiment 1
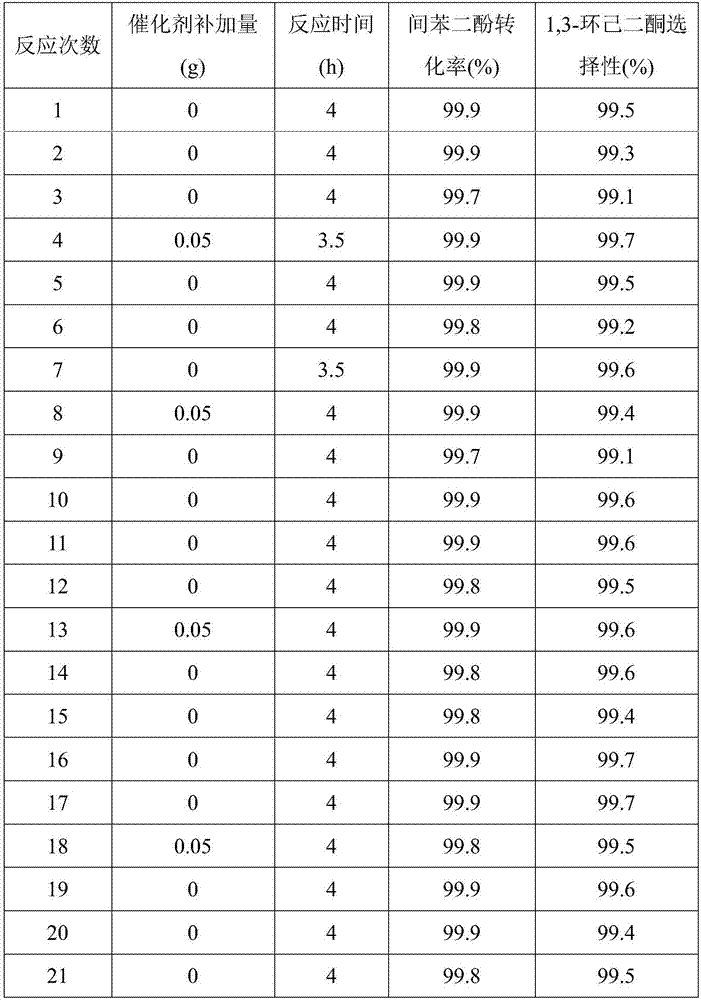
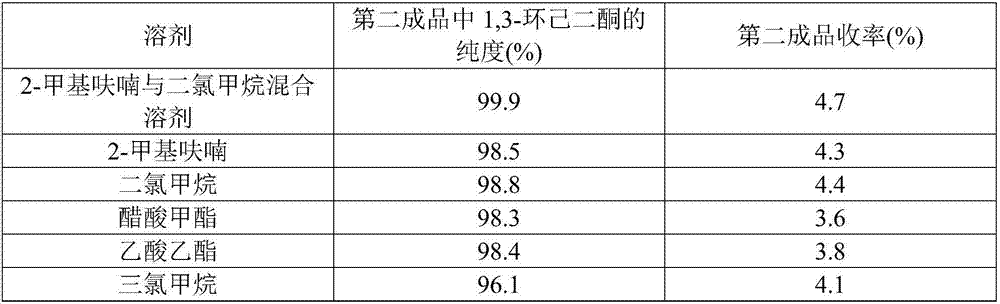
[0043] Add the resorcinol of 100.0g in the 1L autoclave, 29.0g sodium hydroxide, 113.0g water and 0.05gCo-Pd / C catalyst, after covering the still cover, N 2 and H 2 Each displacement three times and with 1.0Mpa of H 2 Test the leak under the atmosphere; if the leak test is qualified, turn on the stirring and adjust the speed to 600-700r / min, and inject H 2 When the pressure is 3.0Mpa, the temperature is raised to 50-60°C. When the reaction absorbs hydrogen, the temperature is controlled at about 60°C, and the H 2 The pressure was maintained at 4.0Mpa until the reaction stopped absorbing hydrogen for about 4 hours. After the reaction, filter out the catalyst, cool the hydrogenated solution to below 40°C, add 60g of 35% hydrochloric acid and freeze and crystallize at 0-5°C, centrifugally filter, and dry to obtain 91.8g of 1,3-cyclohexanedione finished product (the first product) and 208.0 g of centrifuged mother liquor; the yield of the first product 1,3-cyclohexanedione is ...
Embodiment 2
[0045] Other conditions are the same as in Example 1, but the amount of 0.05g Co-Pd / C catalyst is changed to 0.15g. The reaction yielded 94.4g of 1,3-cyclohexanedione finished product (the first product) and 205.5g of centrifuged mother liquor; the yield of the first product 1,3-cyclohexanedione was 92.7%. HPLC detection of the hydrogenation solution shows that the conversion rate of resorcinol is 99.9%, and the selectivity of 1,3-cyclohexanedione is 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com