A kind of liposome preparation of topinastat and preparation method thereof
A technology of liposome preparation and topinostat, which is applied in the field of medicine, can solve problems such as inconvenient administration and limitations of clinical application, and achieve the effects of stable quality, increased route of administration, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
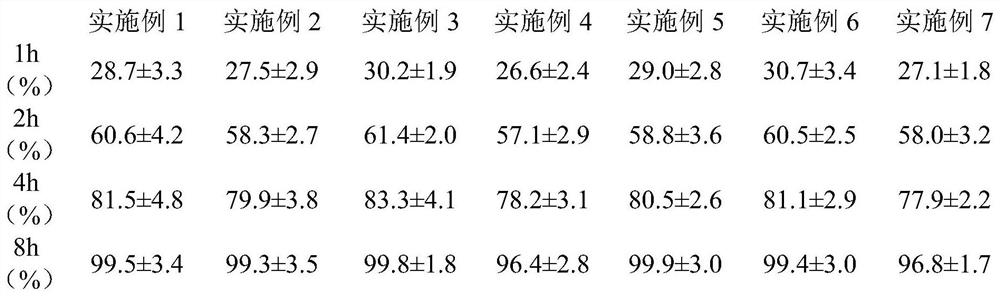
[0029] Take by weighing 90g lecithin, 20g cholesterol and 2g topicastat, add 15mL dichloromethane and make it dissolve completely, evaporate dichloromethane with rotary evaporator, make to form one deck liposome film on the inner wall of round-bottomed flask, residual Put dichloromethane in a desiccator with the lid off to evaporate completely; add 30mL of phosphate buffer solution with pH=7.4 to the flask, and hydrate at 30°C for 2 hours to form an emulsion; use a probe-type ultrasonic instrument to perform ultrasonic treatment for 15 minutes to obtain Liposomes of topinostat. The encapsulation efficiency of topicastat in the liposome was 78.3±3.6%, and the particle size was 549±26nm.
Embodiment 2
[0031] Weigh 50g of distearoylphosphatidylcholine, 45g of cholesterol and 2g of topicastat, dissolve them in 10mL of ether, and slowly inject the resulting solution into the phosphate buffer solution heated to 40°C and stirred by magnetic force through a syringe. The pH of the buffer solution is 6.8, and the obtained suspension is continuously stirred until the ether is removed, and the topirastat liposome is obtained. The encapsulation efficiency of topicastat in the liposome was 84.9±5.1%, and the particle size was 472±34nm.
Embodiment 3
[0033] Weigh 30g of dipalmitoylphosphatidylcholine, 50g of cholesterol and 2g of topicastat, dissolve them in 12mL of ether, and slowly inject the resulting solution into a phosphate buffer heated to 35°C and magnetically stirred through a syringe. The pH of the solution was 7.4, and the resulting suspension was homogenized 3 times by high pressure, and the ether was removed to obtain the topirastat liposome. The encapsulation efficiency of the topicastat in the liposome is 88.0±4.7%, and the particle size is 194±22nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com