Application of Xiyanping injection for preparation of drugs for treating and/or preventing neurodegenerative disease
A neurodegenerative and injection technology, which is applied in the direction of nervous system diseases, drug delivery, drug combination, etc., can solve the problems of no neurodegenerative disease drugs, impurity quality control that cannot meet more precise requirements, and difficulty in preparing liquid preparations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
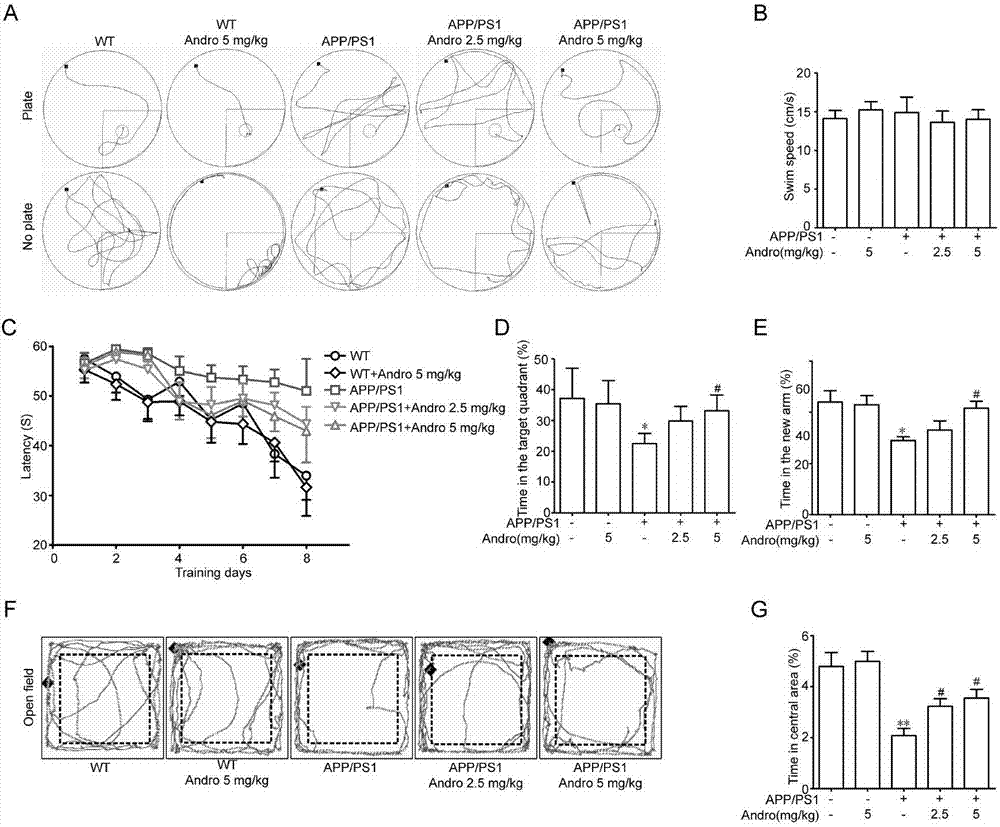
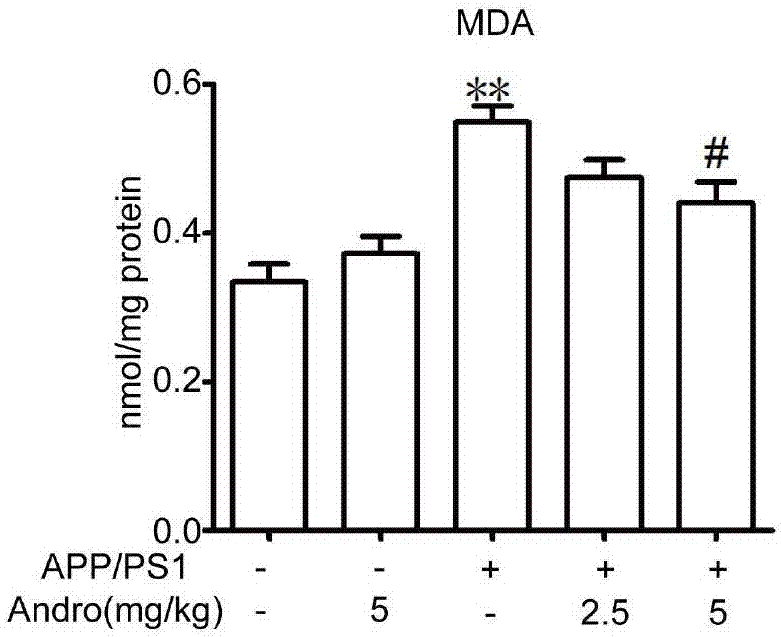
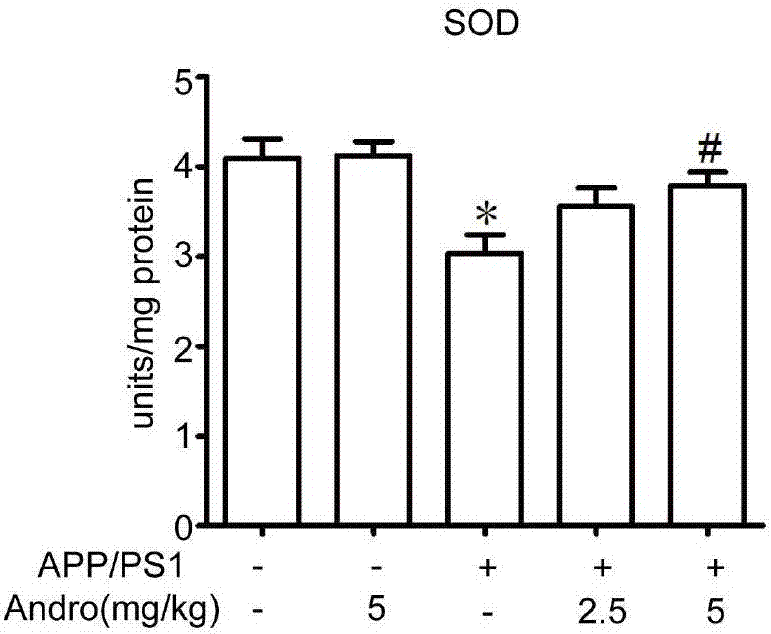
[0043] Example 1: Therapeutic Effect of Xiyanping Injection on APP / PS1 Alzheimer's Disease Mice
[0044] 1. Materials and methods
[0045] 1.1 Experimental animals and drugs
[0046] In this experiment, APP / PS1 male mice and littermate wild-type mice were selected and raised in a quiet environment with room temperature (22°C-24°C), humidity (50%-60%), and 12 hours of light and dark time each day. , free access to food and water. Before the experiment, the animals were acclimated to the experimental environment for 3 days.
[0047] Xiyanping injection was purchased from Jiangxi Qingfeng Pharmaceutical Co., Ltd., and formulated into 2.5mg / kg and 5mg / kg doses.
[0048] 1.2 Animal grouping and administration
[0049] After weighing, the mice were randomly divided into wild type, wild type administration group, APP / PS1 model group, APP / PS1 Xiyanping 2.5 mg / kg and 5 mg / kg treatment groups, with 5 mice in each group.
[0050] 1.3 Behavioral testing
[0051] 1.3.1 Water maze exp...
Embodiment 2
[0078] Example 2: The therapeutic effect of Xiyanping injection on MPTP-induced Parkinson's disease in mice
[0079] 1. Materials and methods
[0080] 1.1 Experimental animals and drugs
[0081] This experiment selects 10-12 week old 25-28g healthy male C57Bl / 6 mice, the mouse is at room temperature (22 ℃-24 ℃), humidity (50%-60%), daily light and dark time each 12h Raised in a quiet environment, free access to food and water. Before the experiment, the animals were acclimated to the experimental environment for 3 days.
[0082] MPTP was purchased from Sigma, and levodopa was purchased from Roche Pharmaceuticals. MPTP was prepared with normal saline within 30 minutes before administration, and stored on ice. Xiyanping injection was purchased from Jiangxi Qingfeng Pharmaceutical Co., Ltd., and was formulated at 5% and 10% administration concentrations, and 0.1ml was injected intraperitoneally into 20g mice .
[0083] 1.2 Animal grouping and administration
[0084] After w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com