Preparation method of 1H-1, 2, 3-triazole
A technology of triazole and 1H-1, which is applied in the field of preparation of 1H-1,2,3-triazole, can solve the problems of low production efficiency, production safety risks and the like, and achieves short production route and high atom utilization rate. , The effect of route economy and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
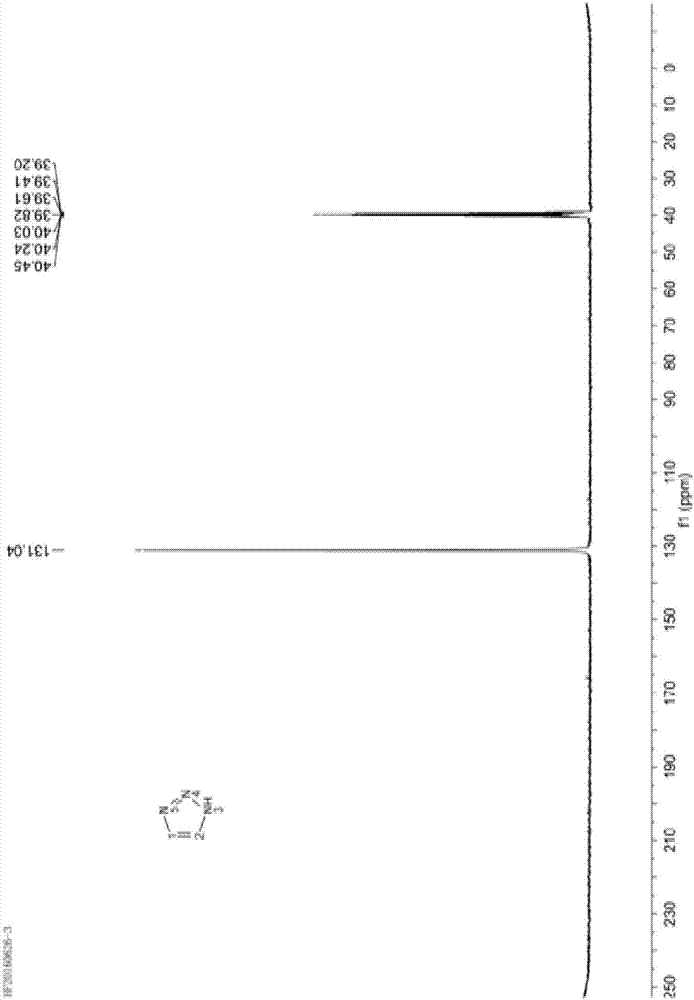
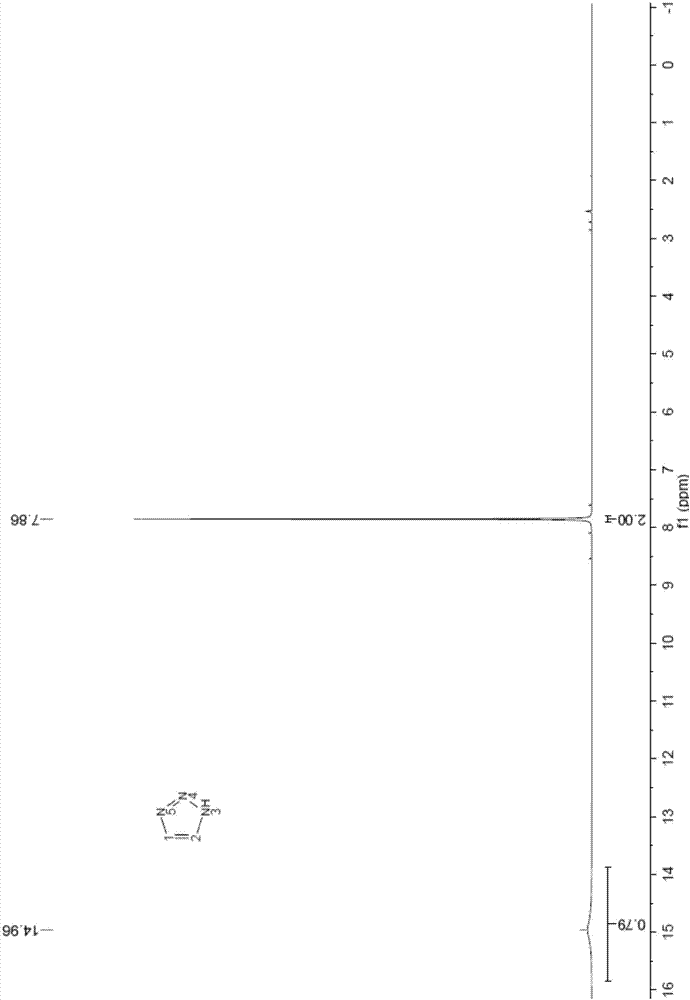
Embodiment 1
[0043] Dissolve 80.0g of sodium nitrite (MW69.00, 1.16mol) in 250g of purified water, stir and dissolve at 30-40°C, then cool to 0-5°C to obtain an aqueous solution of sodium nitrite for use.
[0044] Add 94.5g of 1,2-diaminoethylene hydrochloride (1.00mol) into 250g of glacial acetic acid, stir to dissolve and clarify, cool down to -10~0°C, and add dropwise 330.0g of sodium nitrite solution prepared in advance , temperature control -5 ~ 5 ℃. After dropping, continue to stir and react at 0-5°C for 20 minutes, then slowly raise the temperature to 70-80°C, and keep the reaction for 1 hour.
[0045] After the reaction, concentrate under reduced pressure to recover acetic acid, then cool to 0-5°C, adjust the pH to 8-9 with 10% lye (sodium carbonate aqueous solution), then extract with 800mL dichloromethane several times, and combine the organic layers , concentrated to obtain 68g triazole crude oil.
[0046] Then transfer 68g of triazole crude oil into a 100mL roundness rectific...
Embodiment 2
[0050] Add 94.5g of 1,2-diaminoethylene hydrochloride (1.00mol) into a mixture of 100g of water and 300g of glacial acetic acid, stir to dissolve, cool down to -10~-5°C, and add in batches 85.0g sodium nitrite (MW69.00, 1.23mol), temperature control -10~0℃. After the addition is complete, continue to stir and react at -10 to -5°C for 30 minutes, then slowly raise the temperature to 40 to 50°C, and keep the reaction for 2 hours.
[0051] After the reaction is over, concentrate under reduced pressure to recover acetic acid, add 100g of water, adjust the pH to 9-10 with 10% lye, extract with 1000mL of ethyl acetate, combine the organic layers, and concentrate to obtain 69.5g of triazole crude oil .
[0052] Then transfer 69.5g of triazole crude oil into a 100mL roundness rectification flask, carry out high vacuum column rectification, collect 90~95°C fractions (absolute pressure≤5mmHg), and obtain 64.5g of colorless and transparent liquid 1H-1 , 2,3-triazole, the purity is grea...
Embodiment 3
[0054] After mixing 200g of water and 200g of glacial acetic acid, add 85.0g of sodium nitrite (MW69.00, 1.23mol) under stirring, and after dissolving and clarifying, cool to -10~-5°C. Then, 94.5 g of 1,2-diaminoethylene hydrochloride (1.00 mol) was added to the above reaction solution in small amounts and in batches, the temperature was controlled at 0-5° C., and the addition time was controlled at 0.5-1.0 h. After the addition is complete, continue to stir and react at 0-5°C for 15 minutes, then slowly raise the temperature to 50-60°C, and keep the reaction for 2 hours.
[0055] After the reaction was finished, concentrate under reduced pressure to recover acetic acid, adjust the pH to 9-10 with 10% lye, extract with 1000 mL of dichloromethane in portions, combine the organic layers, and concentrate to obtain 69.0 g of triazole crude oil.
[0056] Then transfer 69.0g of triazole crude oil into a 100mL roundness rectification flask, carry out high vacuum column rectification,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com