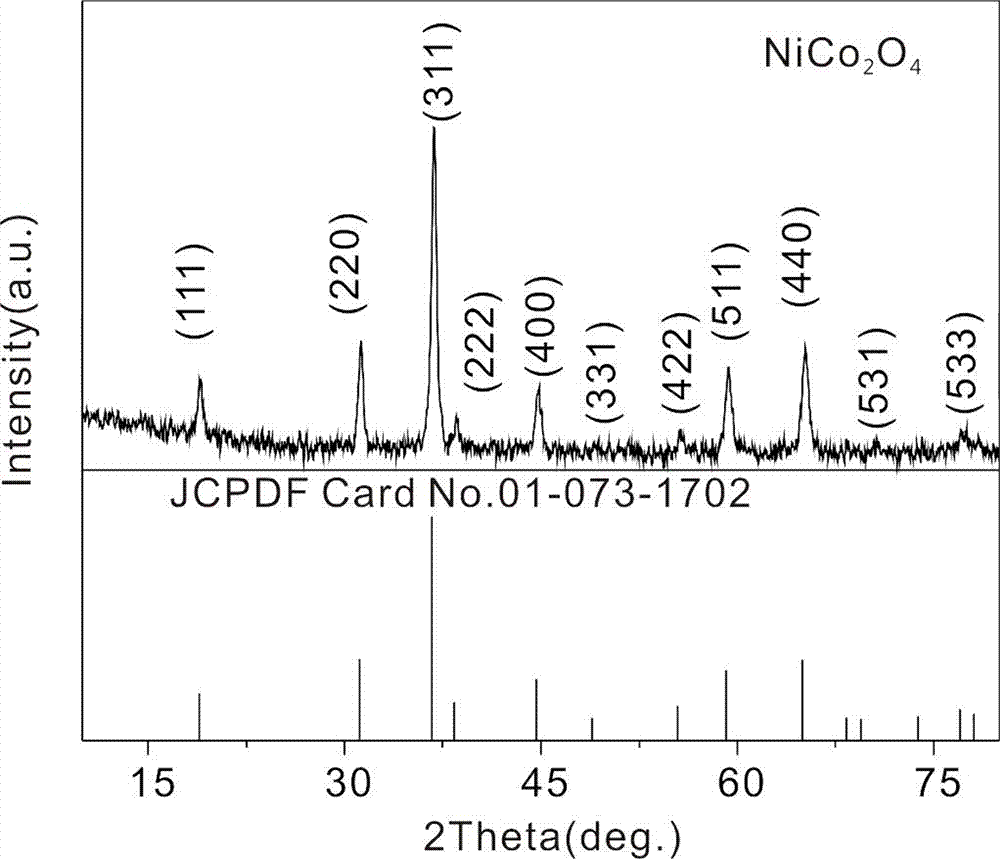
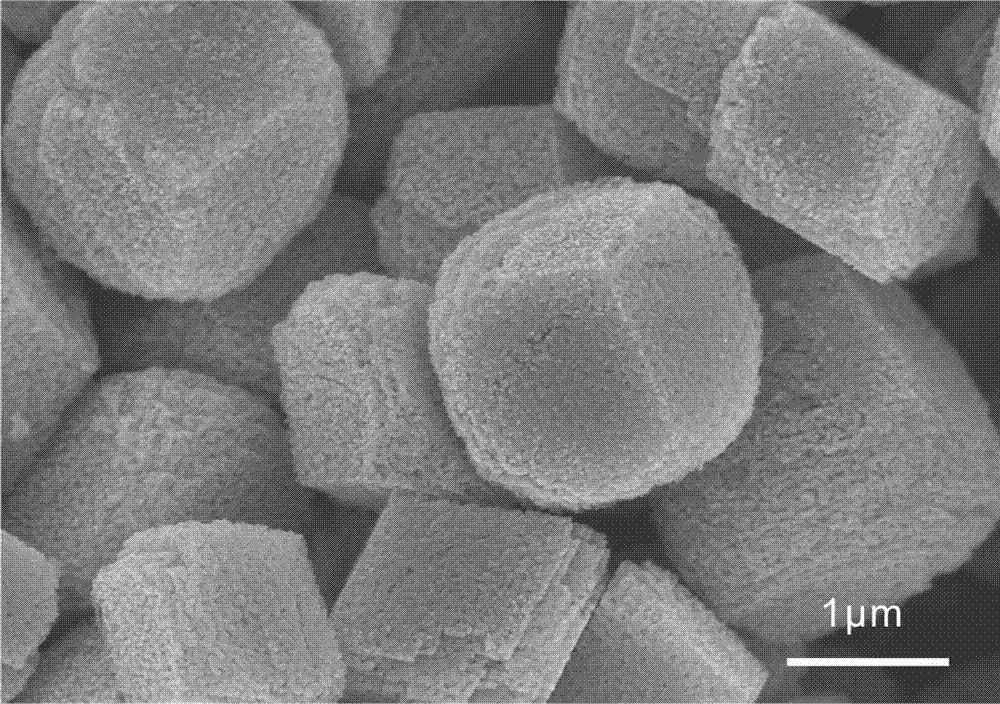
Preparation method of anode material for lithium-ion batteries
A technology for lithium ion batteries and negative electrode materials, which is applied in battery electrodes, secondary batteries, circuits, etc., to achieve the effects of low requirements, excellent cycle stability performance, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Weigh 0.249g nickel acetate tetrahydrate, and 0.498g cobalt acetate tetrahydrate and 3.6g urea, put them into a clean beaker, add 30ml ethylene glycol and deionized water mixed solution (ethylene glycol and deionized water mixed solution The volume ratio is 5:1) and fully stirred into a uniform clear solution;
[0029] (2) Transfer the homogeneously mixed and clear solution in step (1) to a reaction kettle with a polytetrafluoroethylene liner, seal it completely, heat it at a constant temperature of 120°C for 12 hours, take out the liner, and pour off the upper layer of waste liquid , add water and transfer to a centrifuge tube of a designated model for centrifugation, and wash with deionized water and absolute ethanol three times;
[0030] (3) Put the purple sample obtained in step (2) into a drying oven, adjust to 80° C., and dry to obtain a purple solid powder;
[0031] (4) Put the purple solid obtained in step (3) into a fully dried quartz boat, and place it in...
Embodiment 2
[0039](1) Weigh 0.249g nickel acetate tetrahydrate, and 0.498g cobalt acetate tetrahydrate and 3.6g urea, put them into a clean beaker, add 30ml ethylene glycol and deionized water mixed solution (ethylene glycol and deionized water mixed solution The volume ratio is 2:1) and fully stirred into a uniform clear solution;
[0040] (2) Transfer the homogeneously mixed and clear solution in step (1) to a reaction kettle with a polytetrafluoroethylene liner, seal it completely, heat it at a constant temperature of 120°C for 12 hours, take out the liner, and pour off the upper layer of waste liquid , add water and transfer to a centrifuge tube of a designated model for centrifugation, and wash with deionized water and absolute ethanol three times;
[0041] (3) Put the purple sample obtained in step (2) into a drying oven, adjust to 80° C., and dry to obtain a purple solid powder;
[0042] (4) Put the purple solid obtained in step (3) into a fully dried quartz boat, and place it in ...
Embodiment 3
[0044] (1) Weigh 0.249g nickel acetate tetrahydrate, and 0.498g cobalt acetate tetrahydrate and 3.6g urea, put them into a clean beaker, add 30ml ethylene glycol and deionized water mixed solution (ethylene glycol and deionized water mixed solution The volume ratio is 1:1) and fully stirred into a uniform and clear solution;
[0045] (2) Transfer the homogeneously mixed and clear solution in step (1) to a reaction kettle with a polytetrafluoroethylene liner, seal it completely, heat it at a constant temperature of 120°C for 12 hours, take out the liner, and pour off the upper layer of waste liquid , add water and transfer to a centrifuge tube of a designated model for centrifugation, and wash with deionized water and absolute ethanol three times;
[0046] (3) Put the purple sample obtained in step (2) into a drying oven, adjust to 80° C., and dry to obtain a purple solid powder;
[0047] (4) Put the purple solid obtained in step (3) into a fully dried quartz boat, and place i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com