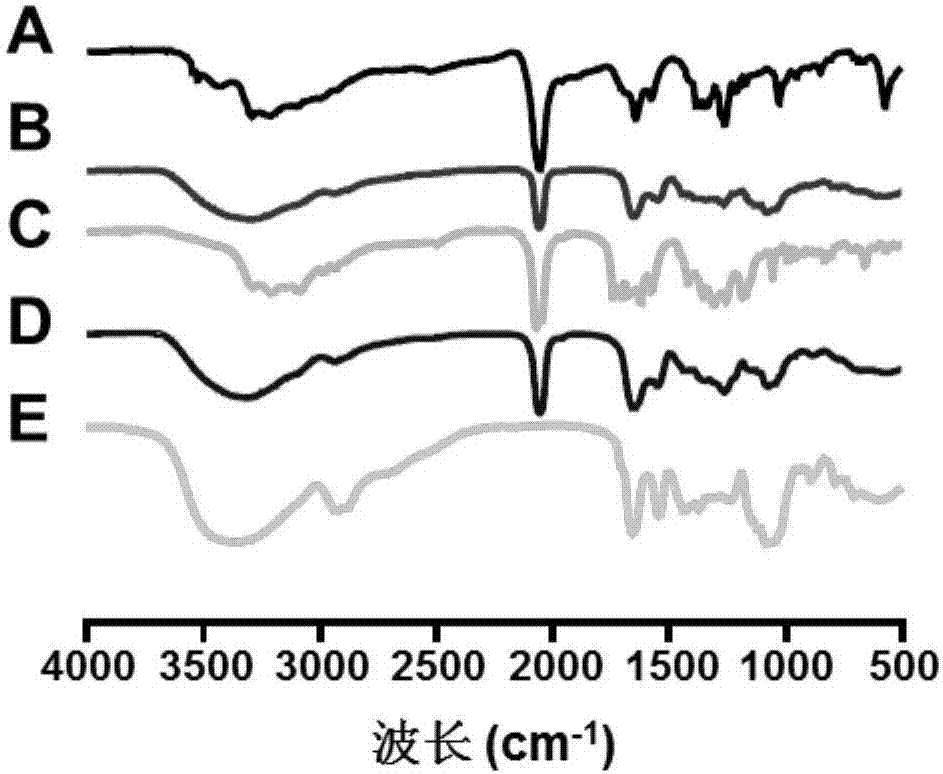
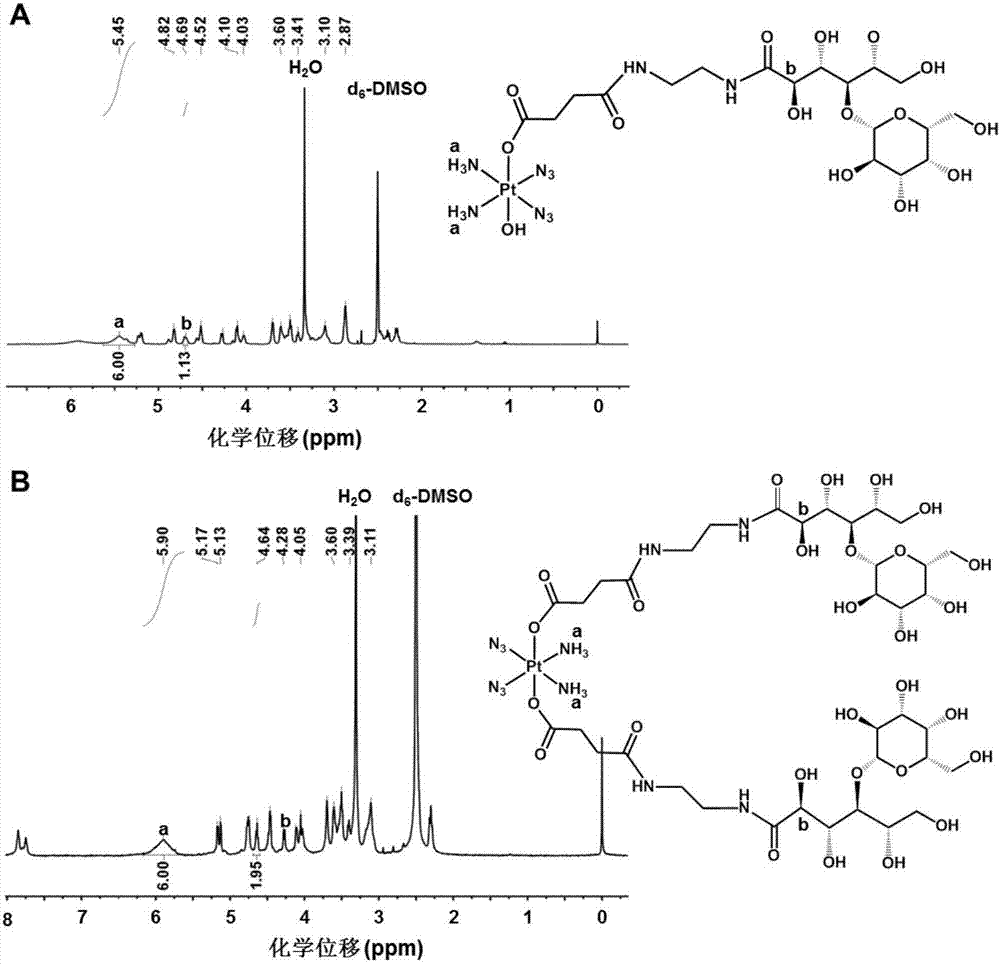
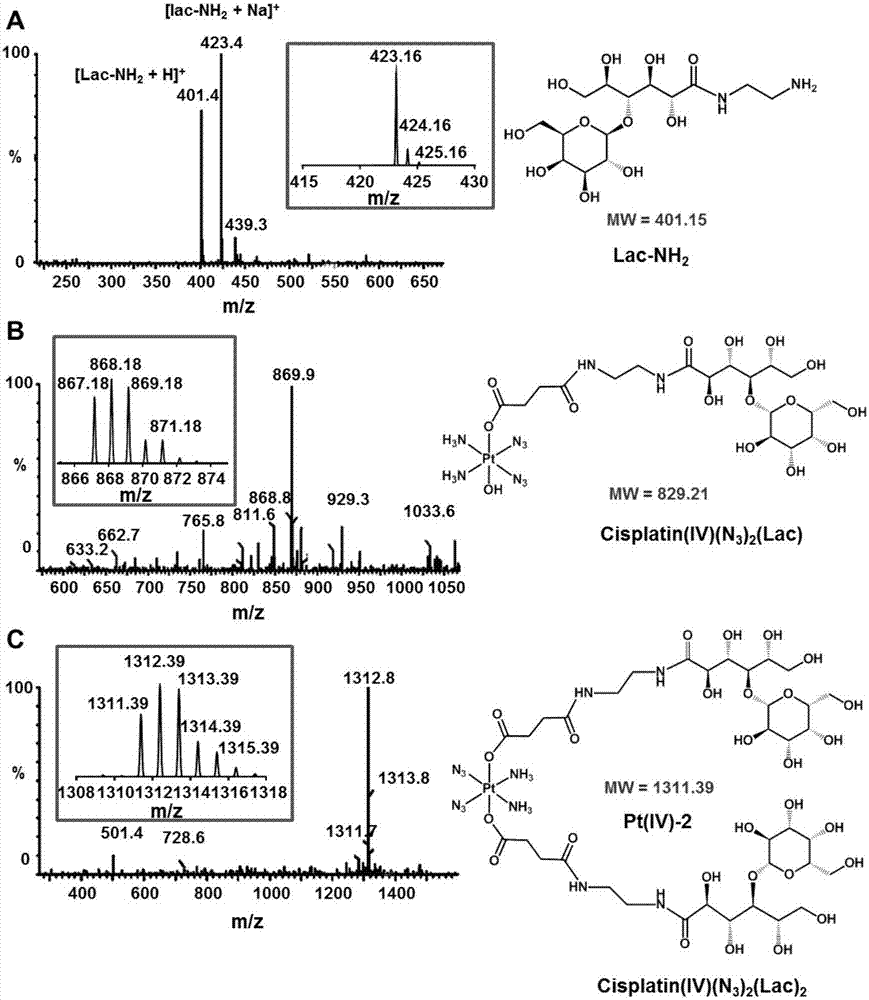
Compound with antitumor activity, and preparation method and application thereof
A technology of anti-cancer activity and compounds, applied in organic active ingredients, medical preparations containing active ingredients, organic chemistry, etc., can solve the problems of poor solubility and curative effect of platinum (IV) compounds, achieve strong killing effect, reduce The effect of toxicity and good killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The present invention also provides a preparation method of a compound with anticancer activity, comprising:
[0038] A) reacting a platinum (II) compound with hydrogen peroxide to obtain a platinum (IV) compound;
[0039] B) reacting the platinum (IV) compound obtained in step A) with succinic anhydride to obtain a platinum (IV) complex containing one or two carboxyl groups in the axial direction;
[0040] C) dehydrating lactobionic acid in a reaction solvent to obtain lactone;
[0041] D) reacting the lactose lactone obtained in step C) with ethylenediamine to obtain lactose, ie, lactosamine, obtained by functionalizing the amino group;
[0042] E) reacting the platinum (IV) complex obtained in step B) with the lactosamine obtained in step D) in the presence of a condensing agent to obtain compounds represented by the structures of formulas (I) and (II);
[0043] According to the present invention, the compound represented by the formula (I) provided by the present in...
Embodiment 1
[0079] The compound shown in formula (I) structure is synthesized
[0080] Cisplatin (600 mg, 2 mmol) was placed in a reaction flask, 10 mL of 30% hydrogen peroxide was added, and after stirring for 5 h at room temperature in the dark, the hydrogen peroxide was removed by filtration to obtain Cisplatin (IV) (NH 3 ) 2 -(OH) 2 yellow powder.
[0081] The resulting Cisplatin(IV)(NH 3 ) 2 -(OH) 2 (334mg, 1mmol) and succinic anhydride (100mg, 1mmol) were dispersed in a dry DMSO solvent, and the reaction was fully stirred at 30°C for 12h, then settled with ether, filtered to obtain a solid and vacuum-dried to obtain a light yellow solid powder Cisplatin(IV)(NH3 ) 2 -(OH)(COOH).
[0082] Disperse lactobionic acid (1g) in 40mL of methanol, add 0.1mL of trifluoroacetic acid, dehydrate at 65°C for 8h to form lactose lactone, drain the methanol, dissolve the lactose lactone in 40mL of methanol, add 10mL of ethylenediamine to react at room temperature for 24h , and then settled wi...
Embodiment 2
[0087] The compound shown in formula (I) structure is synthesized
[0088] Put cisplatin (600mg, 2mmol) in the reaction flask, then add twice the equivalent of silver nitrate (679.48mg, 4mmol), react at 65°C for 12h, then filter the white precipitate, and add 4 times the equivalent of azide to the filtrate Sodium (520mg, 8mmol), react at room temperature for 4 hours, filter to obtain the filter cake, disperse with 10mL of water, add 10mL of 30% hydrogen peroxide, stir at room temperature for 8h, remove the hydrogen peroxide by filtration to obtain Cisplatin (IV) (N 3 ) 2 -(OH) 2 yellow powder.
[0089] The resulting Cisplatin(IV)(N 3 ) 2 -(OH) 2 (347mg, 1mmol) and succinic anhydride (100mg, 1mmol) were dispersed in dry DMSO, and the reaction was fully stirred at 35°C for 12h, then settled with ether, filtered to obtain a solid and vacuum-dried to obtain a light yellow solid powder Cisplatin (IV)(N 3 ) 2 -(OH)(COOH).
[0090] Disperse lactobionic acid (1g) in 40mL of m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com