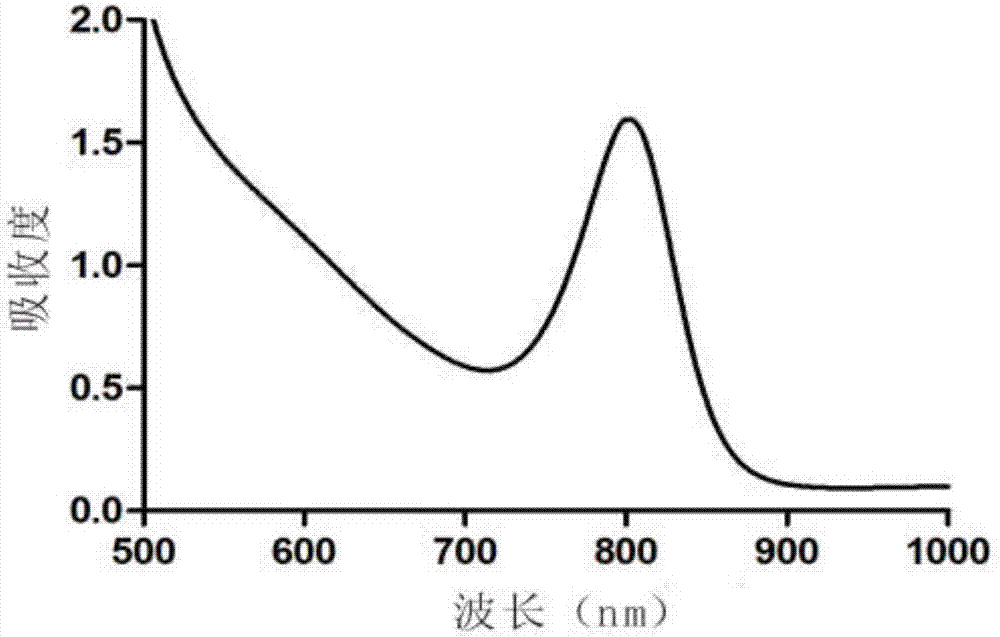
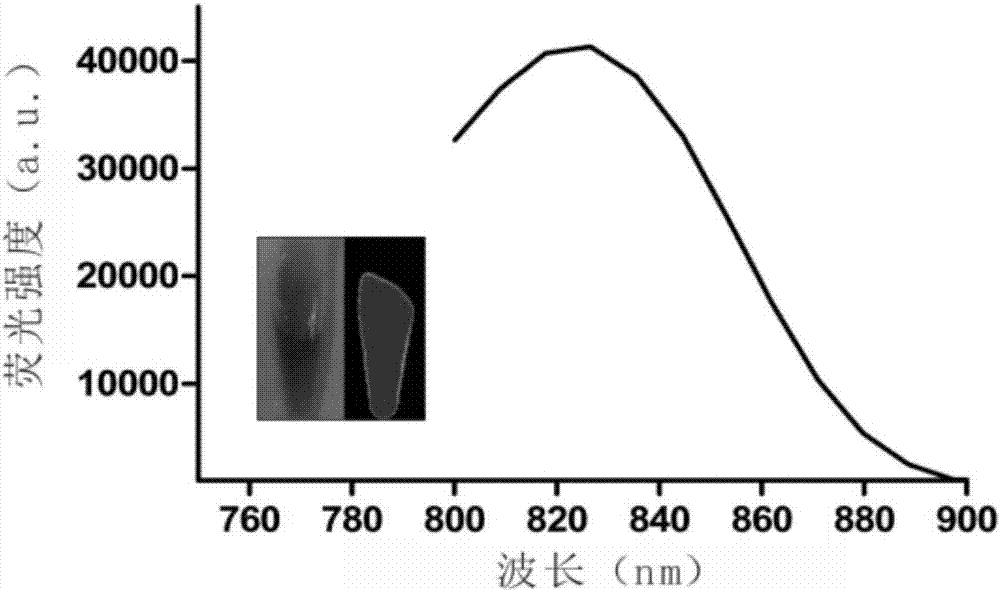
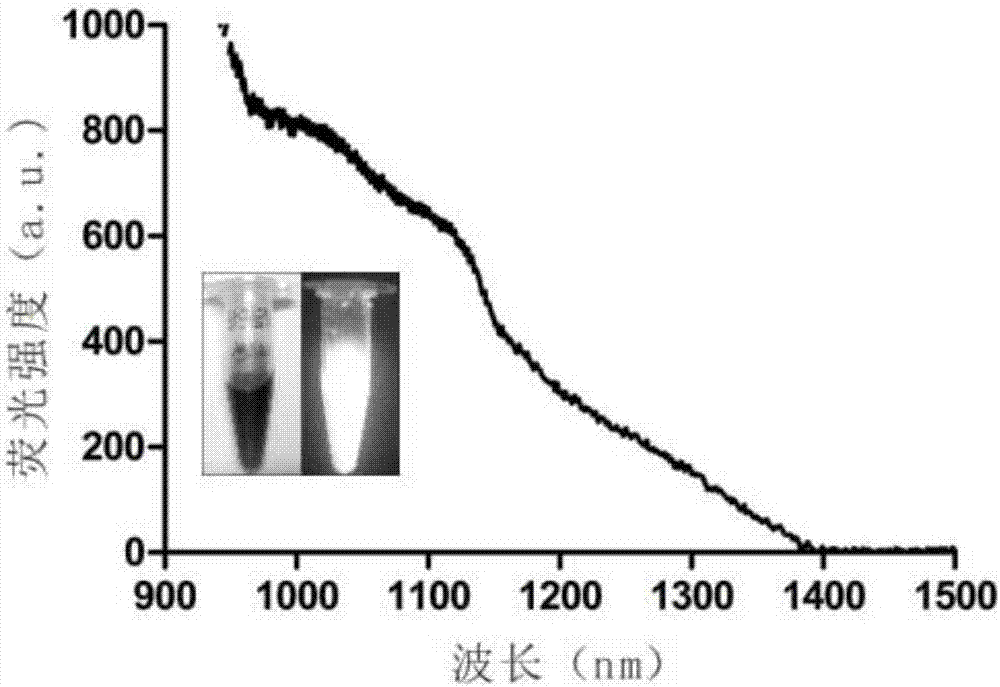
Synthesis method of near infrared silver sulfide quantum dots
A synthesis method and technology of silver sulfide, applied in the direction of chemical instruments and methods, pharmaceutical formulations, preparations for in vivo tests, etc., can solve the problems of simple, green, high-yield, high-efficiency, increase the difficulty of synthesis of silver sulfide quantum dots, Problems such as changes in the original properties of quantum dots, to achieve the effect of simple green synthesis, good biocompatibility, and stable optical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The invention provides a kind of synthetic method of near-infrared silver sulfide quantum dot, and described synthetic method comprises the following steps:
[0043] 1) Dissolve the silver nitrate crystals in the polar solvent ethylene glycol, and stir until dissolved at 3°C-5°C in the dark to obtain solution A;
[0044] 2) In the state of stirring, add mercaptocarboxylic acid solution to obtain solution B;
[0045] 3) Place the solution B under the condition of 70°C-80°C in the dark and stir, and react for 2min-8min, the solution B gradually turns from colorless to milky white, gradually forming silver sulfide crystal nuclei;
[0046] 4) Increase the reaction temperature from 70°C-80°C to 140°C-150°C, continue the reaction, the solution B gradually changes from milky white to clear brown, forming a stable silver sulfide crystal nucleus;
[0047] 5) rapidly cool to room temperature, terminate the reaction;
[0048] 6) Ultrafiltration to remove the solvent and reaction...
Embodiment 1
[0061] Embodiment 1 proposes a kind of synthetic method of near infrared silver sulfide quantum dot, comprises the following steps:
[0062] 1) Weigh 8.49 mg (0.05 mmol) of silver nitrate crystals and dissolve them in 20 ml of anhydrous ethylene glycol, and stir at 4°C in the dark until the silver nitrate dissolves to obtain 2.5 mmol / L silver nitrate solution A;
[0063] 2) Slowly add 0.05 mmol of 3-mercaptopropionic acid to solution A under stirring to obtain colorless and transparent solution B;
[0064] 3) Put solution B in an oil bath at 75°C, stir evenly for 5 minutes, solution B gradually changes from colorless to milky white;
[0065] 4) At a heating rate of 10°C / min, raise the temperature of the oil bath reaction system from 75°C to 145°C in about 7 minutes, start timing when the temperature reaches 145°C, and continue the reaction for 100 minutes to obtain a clear and translucent brown reaction solution ;
[0066] 5) Rapidly cool down to room temperature in an ice b...
Embodiment 2-3
[0072] Embodiment 2-3 proposes a kind of synthetic method of near-infrared silver sulfide quantum dots respectively, and synthetic procedure is the same as embodiment 1, and difference is: the amount of adding 3-mercaptopropionic acid in step 2) is respectively: 0.075mmol, 0.1 mmol, that is, the feeding ratio of silver nitrate / 3-mercaptopropionic acid is 1:1.5, 1:2 respectively.
[0073] The average particle size, average surface potential and fluorescence emission range of silver sulfide quantum dots obtained in Examples 2-3 are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com