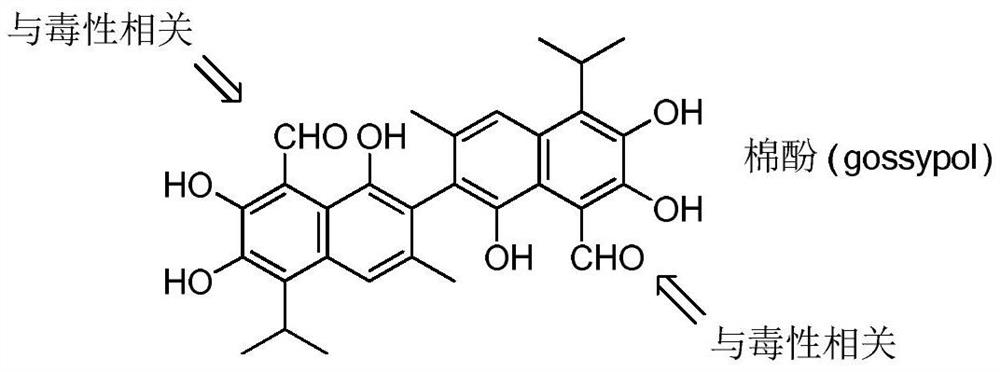
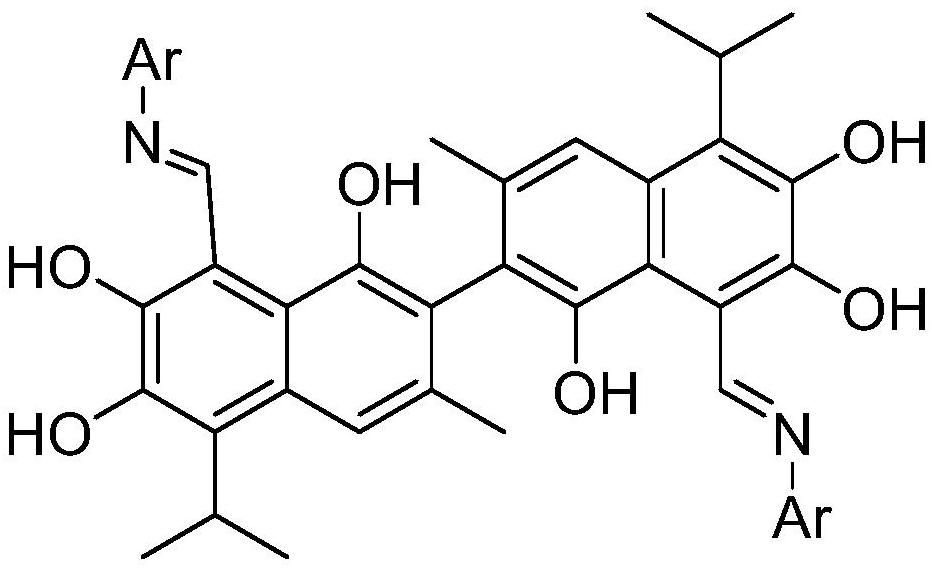
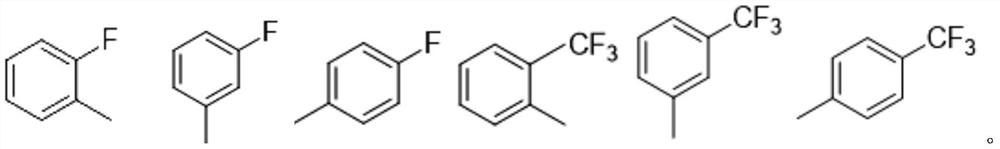
Fluorine-containing gossypol derivatives with antitumor activity, preparation method and application thereof
A technology of fluorine-containing gossypol and anti-tumor drugs, which is applied in the fields of anti-tumor drugs, imino compound preparation, organic chemistry, etc., and can solve the problems of weakening the therapeutic effect of drugs, limiting the maximum tolerated dose of gossypol, and increasing toxicity , to achieve significant in vitro inhibitory effect, low toxicity, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The preparation method of above-mentioned fluorine-containing gossypol derivative comprises the following steps:
[0026] (1) get gossypol and dissolve in methanol;
[0027] (2) Add one of 2-fluoroaniline, 3-fluoroaniline, 4-fluoroaniline, 2-trifluoromethylaniline, 3-trifluoromethylaniline or 4-trifluoromethylaniline, heat and stir at constant temperature , Refluxed, a yellow solid was precipitated and filtered. Preferably, the stirring is magnetic stirring. Preferably, the temperature is 60-70° C. during stirring, and the mixture is heated, stirred and refluxed for 3-5 hours.
[0028] (3) Wash with petroleum ether-ethyl acetate solution, and recrystallize with petroleum ether-ethyl acetate. Preferably, the volume ratio of petroleum ether-ethyl acetate used for washing is 14-18:1. Preferably, the recrystallization method is as follows: dissolve the fluorine-containing gossypol derivative with ethyl acetate in a water bath at 50-70°C, then drop petroleum ether to sat...
Embodiment 1
[0030] Embodiment 1 (gossypol derivative 3a)
[0031] 8,8'-bis((E)-(2-fluorophenylimino)methyl)-5,5'-diisopropyl-3,3'-dimethyl-2,2'-binaphthyl-1,1',6,6', 7,7'-h exaol
[0032]
[0033] Steps: Take 0.5789g (1mmol) of gossypol, add 40ml of methanol to dissolve, add 0.45ml (4mmol) of 2-fluoroaniline, add a stirrer, place the reaction device in a collector type constant temperature heating magnetic stirrer, and adjust the temperature to Heat, stir and reflux at 65°C for 4 hours, a yellow solid precipitates, filters, washes with petroleum ether-ethyl acetate (16:1), and recrystallizes with petroleum ether-ethyl acetate.
[0034] The specific method of recrystallization: dissolve the fluorine-containing gossypol derivative 3a with ethyl acetate in a 60°C water bath, then slowly drop petroleum ether to saturate the solution, and let it cool to room temperature. After 3 days, yellow crystals precipitate out.
[0035] Get 0.61g of fluorine-containing gossypol derivative 3a product...
Embodiment 2
[0036] Embodiment 2 (gossypol derivative 3b)
[0037] 8,8'-bis((E)-(3-fluorophenylimino)methyl)-5,5'-diisopropyl-3,3'-dimethyl-2,2'-binaphthyl-1,1',6,6', 7,7'-h exaol
[0038]
[0039] Steps: Take 0.5789g (1mmol) of gossypol, add 40ml of methanol to dissolve, add 0.45ml (4mmol) of 3-fluoroaniline, add a stirrer, place the reaction device in a collector type constant temperature heating magnetic stirrer, and adjust the temperature to Heat, stir and reflux at 65°C for 4 hours, a yellow solid precipitates, filters, washes with petroleum ether-ethyl acetate (16:1), and recrystallizes with petroleum ether-ethyl acetate.
[0040] The specific method of recrystallization: dissolve the fluorine-containing gossypol derivative 3b with ethyl acetate in a 60°C water bath, then slowly drop petroleum ether to saturate the solution, and let it cool to room temperature. After 3 days, yellow crystals precipitate out.
[0041] Obtain 0.58g of fluorine-containing gossypol derivative 3b prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com