Method for determining dissolution rate of DMSA (Dimercaptosuccinic Acid) preparation
A dimercaptosuccinic acid and dissolution technology, which is applied in the field of medical testing, can solve problems such as defects in quality standards and the inability to guarantee the effectiveness of clinical medication, and achieve high sensitivity, improve quality control standards, and improve quality controllability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1.1 Chromatographic conditions: adopt Kromasil C-18 chromatographic column, particle diameter is 5um, column length is 150mm, column diameter is 4.6mm, LC Solution chromatographic workstation, with acetonitrile as mobile phase A, with 0.05mol / L potassium dihydrogen phosphate aqueous solution ( Phosphoric acid was used to adjust the pH value to 2.5) as mobile phase B, the ratio of mobile phase A to B was 10:90, the detection wavelength was 210nm, the column temperature was 30°C, and the flow rate was 1.0mL / min.
[0042] 1.2 Preparation of dissolution medium
[0043] Sodium lauryl sulfate, sodium bisulfite are placed in water, constant volume, obtain 1000mL stripping medium, the mass concentration of sodium lauryl sulfate in the stripping medium is 0.2%, the mass concentration of sodium bisulfite in the stripping medium 0.1%.
[0044] 1.3 Preparation of test solution and control solution
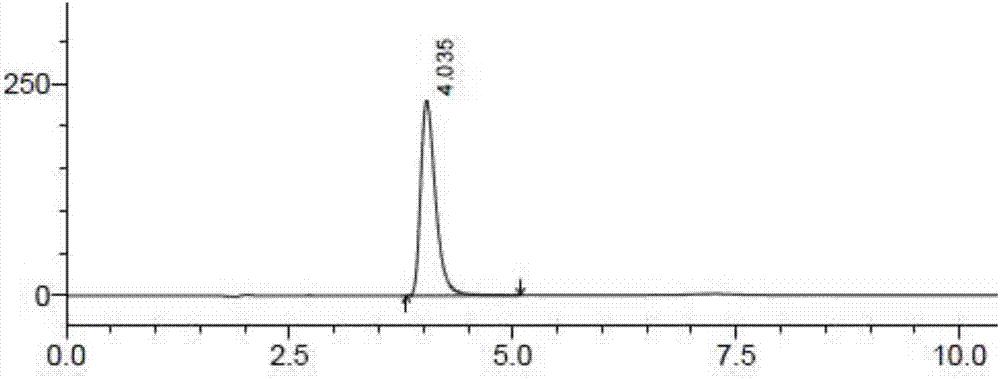
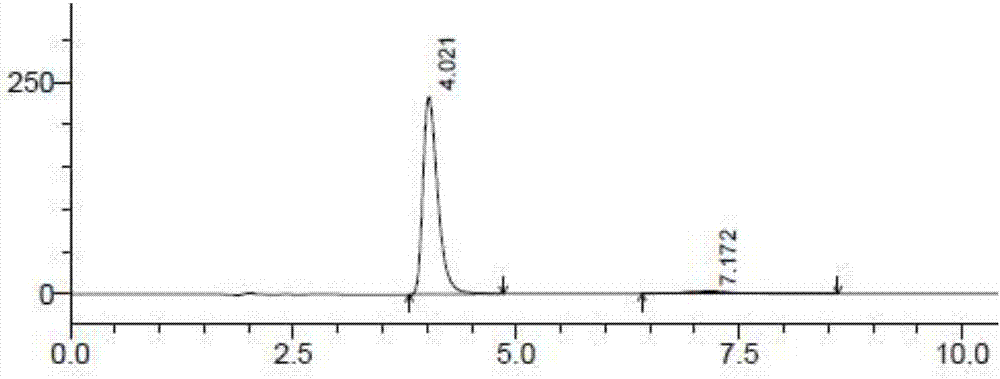
[0045] Accurately weigh 25 mg of the contents of the dimercaptosuccinic acid cont...
Embodiment 2
[0055] 2.1 Chromatographic conditions are the same as 1.1.
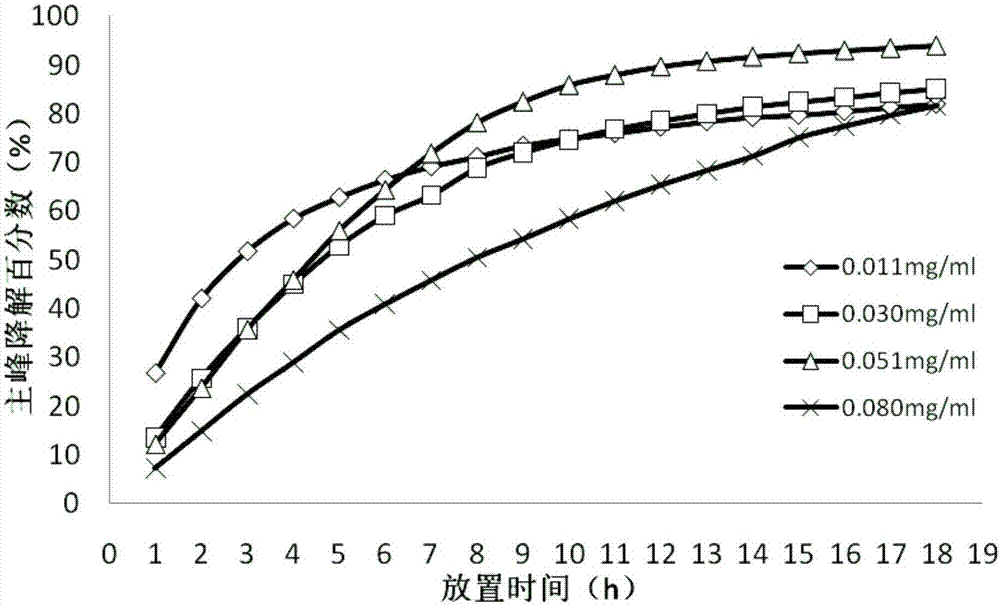
[0056] 2.2 According to the description in the 2015 edition of the Chinese Pharmacopoeia, dimercaptosuccinic acid is slightly soluble in methanol or ethanol, and almost insoluble in water or chloroform. Experimental investigation of the solubility of dimercaptosuccinic acid in water, mass concentration of 0.1% sodium lauryl sulfate aqueous solution, mass concentration of 0.2% sodium lauryl sulfate aqueous solution, pH4.5 and pH6.8 phosphate buffer , the results are shown in Table 2 below.
[0057] Table 2 raw material solubility test result
[0058] solvent
Solubility (mg / mL)
Whether the sink condition is met
water
0.2
no
0.05% sodium lauryl sulfate water soluble
0.5
no
0.1% sodium lauryl sulfate aqueous solution
0.8
yes
pH4.5 phosphate buffered saline solution
1.0
yes
pH6.8 phosphate buffered saline solution
0.3
no
[0059]...
Embodiment 3
[0062] 3.1 Chromatographic conditions are the same as 1.1.
[0063] 3.2 Preparation of dissolution medium
[0064] Sodium lauryl sulfate is placed in water, constant volume, obtains 1000mL dissolution medium, and the mass concentration of sodium lauryl sulfate in the dissolution medium is 0.2%.
[0065] 3.3 Take dimercaptosuccinic acid capsules (1 capsule, the specification is 50mg / capsule), according to the dissolution test method (Chinese Pharmacopoeia 2015 Edition Four General Rules 0931 First Method), add 1000mL of the dissolution medium obtained in 3.2, and the rotation speed is 100r / min, operate according to the law, after 45min, take 5-8mL of the solution, filter, and take the subsequent filtrate as the test solution.
[0066] Another dimercaptosuccinic acid was taken as a control, dissolved in methanol, and then diluted with a dissolution medium to prepare a solution containing 50 μg of dimercaptosuccinic acid per 1 mL, as a control solution. Precisely measure 20 μL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com